ChBE assistant professor, a computational chemical engineer, to advance fundamental understanding of promising approach for safe, streamlined, cost-effective biomass and CO2 conversion
Is it possible to build safe, sustainable chemical plants on a small scale? The kind of plants that could—among other things—convert biomass into biofuel, on the very farms producing those crops?
Possibly. But doing so requires figuring out, in part, a more benign process of hydrogenation, the chemical reaction between molecular hydrogen and other compounds and elements that is used to create new molecules.
Srinivas Rangarajan, an assistant professor of chemical and biomolecular engineering in Lehigh University’s P.C. Rossin College of Engineering and Applied Science, recently won support from the National Science Foundation’s Faculty Early Career Development (CAREER) program for his proposal to develop novel tools to better understand a promising chemistry called catalytic transfer hydrogenation (CTH).
The prestigious NSF CAREER award is given annually to junior faculty members across the U.S. who exemplify the role of teacher-scholars through outstanding research, excellent education, and the integration of education and research. Each award provides stable support at the level of approximately $500,000 for a five-year period.
When hydrogenation is used to make ultra-low-sulfur diesel, for example, it requires a huge catalytic reactor, temperatures of up to 500 degrees Celsius, and hundreds of pounds of pressure.
Similar chemistry could be used at a much smaller scale to do things like process biomass, upcycle plastic waste, or manufacture specialty chemicals. But such distributed processing would require reactors that are much smaller and operate at significantly lower temperatures and pressures.
And that means using a different hydrogen source.
“The problem with molecular hydrogen is that it’s very light,” says Rangarajan. “To transport it, you need to compress it using very high pressures. And to use it, you need very high pressures. But if you can use a different molecule that can carry hydrogen chemically, that molecule could be used as a hydrogen donor. It will decompose or get converted, and in the process, lose hydrogen, which then becomes available for something like biomass conversion.”
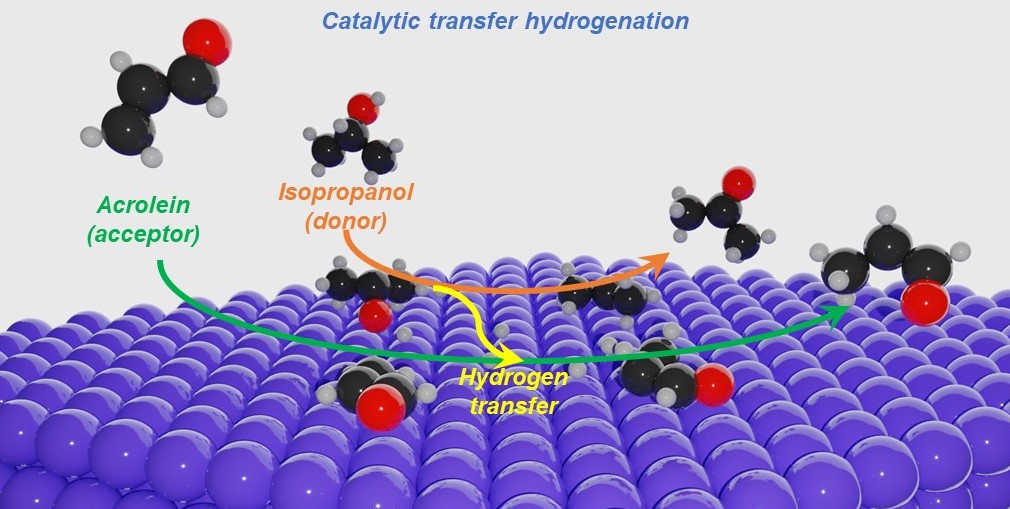 The process of transferring hydrogen atoms from a hydrogen donor to a hydrogen acceptor is called catalytic transfer hydrogenation. “So instead of using a molecular hydrogen, you’re using a liquid molecule that can be shipped very easily from where it’s produced to wherever it’s needed,” he explains. “It can then be used at near-ambient temperatures, and at one atmospheric pressure. So in that sense, CTH could lead to a more compact, safe, modular process.”
The process of transferring hydrogen atoms from a hydrogen donor to a hydrogen acceptor is called catalytic transfer hydrogenation. “So instead of using a molecular hydrogen, you’re using a liquid molecule that can be shipped very easily from where it’s produced to wherever it’s needed,” he explains. “It can then be used at near-ambient temperatures, and at one atmospheric pressure. So in that sense, CTH could lead to a more compact, safe, modular process.”
While that’s the big-picture view, Rangarajan’s focus is on the molecular level. He will develop a novel computational framework to answer two fundamental questions: How does this hydrogen transfer work exactly at the molecular scale, and what’s the right donor and the right catalyst for a given acceptor?
“I’m using a model compound called acrolein as my hydrogen acceptor,” he says. “It has functionalities representative of many biomass compounds and many unsaturated chemicals that come up in the chemicals industry, for example, oleochemicals, solvents, and pharmaceuticals. To hydrogenate acrolein, I need to find out what is the right molecule to supply the hydrogen, and what is the right catalyst that can do this chemistry selectively, that is, without making any undesired byproducts.”
To address possible combinations of donors and catalysts that number in the tens of thousands, Rangarajan will build on his prior work, as well as his recent work at Lehigh, to create a technique called high-throughput kinetic modeling. It incorporates quantum chemistry, optimization, and machine learning to build models that will provide data on how different parameters affect performance.
“This technique allows us to determine how the parameters affect the speed of the reaction and whether the desired product will actually be formed,” he says. “This hasn’t been done before.”
The ultimate goal is to develop processes that are more energy efficient. The potential impact could be huge, he says, given that 45 million tons of hydrogen are used globally every year to make chemicals and energy carriers like fuels. Alternatives to molecular hydrogen could reduce operating expenses for plants by reducing infrastructure, storage, and transportation costs.
But more immediately, where economies of scale don’t work, “like for a farm looking to process its biomass or a specialty chemical company that doesn’t have large production requirements, the main idea is you’re looking for process intensification,” he says. “Smaller, cost-effective, safe solutions that are energy and carbon efficient.”
The CAREER funding will also support educational outreach around what Rangarajan calls “computational thinking” in both undergraduate and graduate students. He’s developing a range of activities, including research projects in software development, a new course in data science for chemical engineers, and experiential learning opportunities through Lehigh’s Office of Creative Inquiry focused on building interactive data-visualization models. He’ll also assist area high school teachers interested in programming or modeling.
“Computations and data science are becoming more and more mainstream in chemical engineering,” he says. “We’re increasingly using mathematics, mathematical modeling, and data to solve problems in industry and academia. I’m really looking forward to promoting algorithmic thinking in the next generation of scientists and engineers.”
About Srinivas Rangarajan
Srinivas Rangarajan is an assistant professor of chemical and biomolecular engineering at Lehigh University. He joined the faculty of the P.C. Rossin College of Engineering and Applied Science in January 2017, after his stint as a postdoctoral scholar at the University of Wisconsin, Madison. He received his B.Tech. (2007) from the Indian Institute of Technology, Madras, and PhD (2013) from the University of Minnesota, both in chemical engineering. His industrial experience includes previous employment (2007-2008) at Shell Global Solutions in the Netherlands and India as a senior associate technologist in hydroprocessing.
Research in the Rangarajan group is at the intersection of heterogeneous catalysis, materials science, and process systems engineering. The group develops and applies a variety of computational tools to model and design catalytic systems and materials that are of relevance in energy and environment and are governed by complex chemistries. The spectrum of tools include electronic structure calculations using density functional theory (DFT), microkinetic modeling, optimization, cheminformatics, automated mechanism generation, and machine learning.
Rangarajan has published over 35 peer-reviewed articles, including a dozen since joining Lehigh, in reputed journals such as ACS Catalysis, Applied Catalysis B, Accounts of Chemical Research, and Nature Communications. His recent awards and honors include the David Smith Graduate Publication Award from the American Institute of Chemical Engineers (CAST division), P.C. Rossin Assistant Professorship, and John Ochs Faculty Achievement Award from the Baker Institute of Creative Inquiry. His group has been supported by grants from the NSF, ACS-PRF (Doctoral New Investigator award), and the Commonwealth of Pennsylvania (PITA).

