Researcher aims laser beams at fruit flies in the fight against heart disease
The mysteries of the human heart are many, but Lehigh University's Chao Zhou is aiming to unlock at least one of them -- and possibly improve pacemaker technology in the process – by way of laser beams trained upon the torsos of fruit flies.
In October, Zhou, assistant professor of electrical and computer engineering, became the first Lehigh researcher to have a paper published in the new science journal, Science Advances. Written in collaboration with the Massachusetts General Hospital and Harvard Medical School, “Optogenetic pacing in Drosophila melanogaster” explores the application of light, or optogenetics, as a possible alternative to artificial pacemakers. Through this project Zhou and his team observe the effect of lasers on the heart rates of Drosophila melanogaster, more commonly known as fruit flies.
In addition, Zhou has been the recipient of a 3-year, half-million dollar grant from the National Institute of Biomedical Imaging and Bioengineering to continue building on the project and exploring different pacing strategies.
Two technologies, beating in harmony
According to Zhou, there are two major long-term impacts from the research.
“One is straightforward,” he says. “This project could lead to an optical pacemaker for human patients, but this admittedly has a long way to go. The second seems more achievable in the short term – studying and identifying causes and mechanisms of genes associated with heart disease in order to develop novel therapies to combat it.”
Despite the widespread use and acceptance of electrical pacemakers, these devices can be associated with a variety of risks. As with many surgical procedures, the implant process is an invasive one that can be fatal if not successful, and pacemakers must be replaced on a regular basis. Furthermore, the electrical stimulation isn’t always guaranteed to target solely the heart. Pulses sent out may result in contractions from other areas of the chest, causing discomfort. Consequently, optical pacemakers may potentially be a safer and more convenient substitute.
“If this is successful, then we could test it on larger animals and eventually 20 years down the road there might be no need to insert electrodes [in people],” says Zhou.
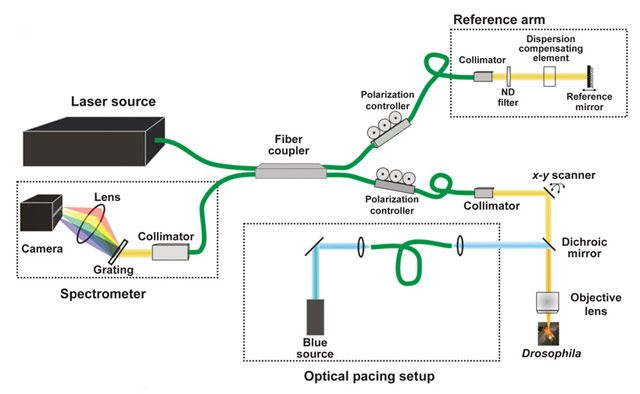
Equally as important as the research performed is the instrument used in the procedure. While traditional microscopes require the specimens to be fixed and prepared prior to observation, the microscope that Zhou’s developed utilizes optical coherence tomography to preserve the specimen’s original form without staining or slicing. Although optical tomography is a non-invasive imaging technique typically used for 3D eye examinations, Zhou has extended the application of its function.
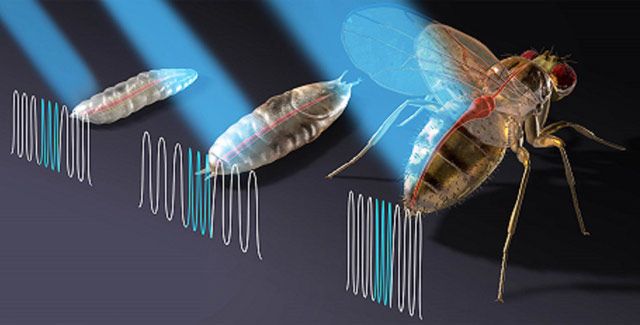
“The article has two components,” Zhou reports. “We are basically combining two technologies. We’re using light to stimulate the fly’s heart -- every time we shine the blue light, the heart contracts. We employ an integrated optical microscope to monitor how the hearts beat with or without the stimulation of the light.”
The integrated microscope and laser used by Zhou’s group are physically connected; in the current project, they used the system to focus on the response of fruit-fly hearts during three stages of the insects’ development over the life-cycle of the specimens.
Illuminating research
The paper has been in the works since late 2012, with two years devoted to polishing the idea and several rounds of trial and error. While a group in Massachusetts General Hospital specializing in gene modification developed the fly models and light-sensitive protein, Harvard sent the flies to Lehigh to perform the stimulation imaging and data analysis.
Zhou received his PhD in physics from the University of Pennsylvania with a focus in optics and developing optical technology for biomedical applications. During postdoctoral training at the Massachusetts Institute of Technology, he worked under a mentor who pioneered optical coherence tomography, and along the way established a collaboration with Harvard. After joining the Lehigh faculty in the summer of 2012, Zhou continued his Harvard collaboration.
“We’d already worked together and built a solid foundation,” Zhou says. “When I came to Lehigh, I started to think about how we could take this work in a new direction. I knew we could see the heart non-invasively, but I always felt like that wasn’t enough – that there were more exciting opportunities to be uncovered.”
In 2012, Zhou noticed developments in the field of optogenetics, where proteins are being inserted into animals to express light-sensitive proteins. Initially, the technique was used to stimulate neurons, using light as a controller.
“I was thinking that if it works with neurons, and if you could sufficiently stimulate heart muscles to cause contractions, optical control of the heart was feasible,” he recalls.
The work covered in Science Advances marks the first time Zhou has demonstrated that this technique could be non-invasively performed on fruit flies using an integrated microscope with a low power and completely safe laser apparatus.
-Nicole Schor is a journalism student interning with the P.C. Rossin College of Engineering and Applied Science.