Articular cartilage exists where bones meet at the joints. This type of cartilage provides a cushioning material to protect the ends of bones and is tightly integrated with bone through a gradient region known as the osteochondral interface―osteo means related to bone, chondral related to cartilage. When articular cartilage is absent or damaged, debilitating pain results.
Unlike some tissues, cartilage cannot regenerate. It lacks blood vessels to support such repair. After injury or damage, cartilage degeneration progresses, leading to osteoarthritis, which affects approximately 27 million Americans.
“Medical intervention is the only way to regenerate osteochondral tissue,” says Lesley Chow, an assistant professor of materials science and engineering and bioengineering who is also affiliated with Lehigh's Institute for Functional Materials and Devices (I-FMD). “To successfully regenerate this cartilage and make it functional, we must consider the fact that function is related to both the cartilage and the bone. If the cartilage doesn’t have a good anchor, it’s pointless. You could regenerate beautiful cartilage, but it won’t last if it isn’t anchored to that bone immediately beneath it.”
This presents a huge engineering challenge, says Chow, as it’s difficult to create one organ made up of two very different tissues. What is needed is a tissue engineering method that respects the multi-component and organizational nature of how tissues form in nature, she says, adding: “Then we’d have the ability to create something that’s durable.”
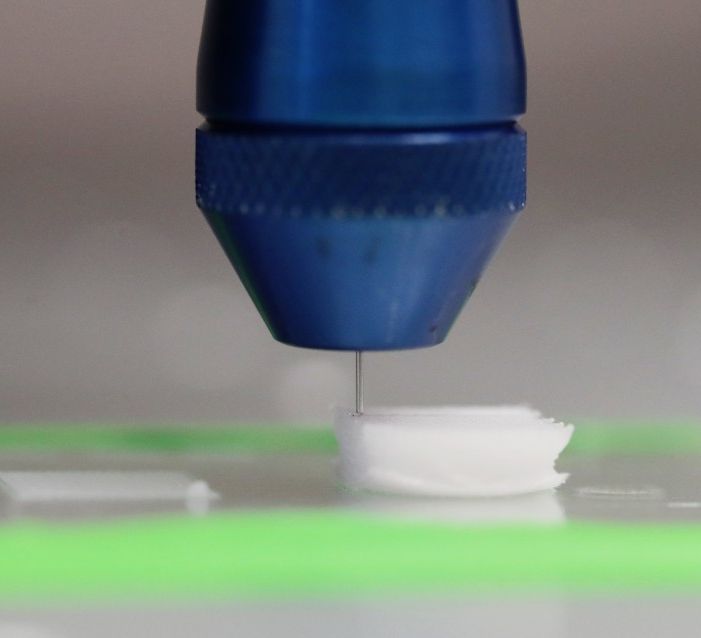
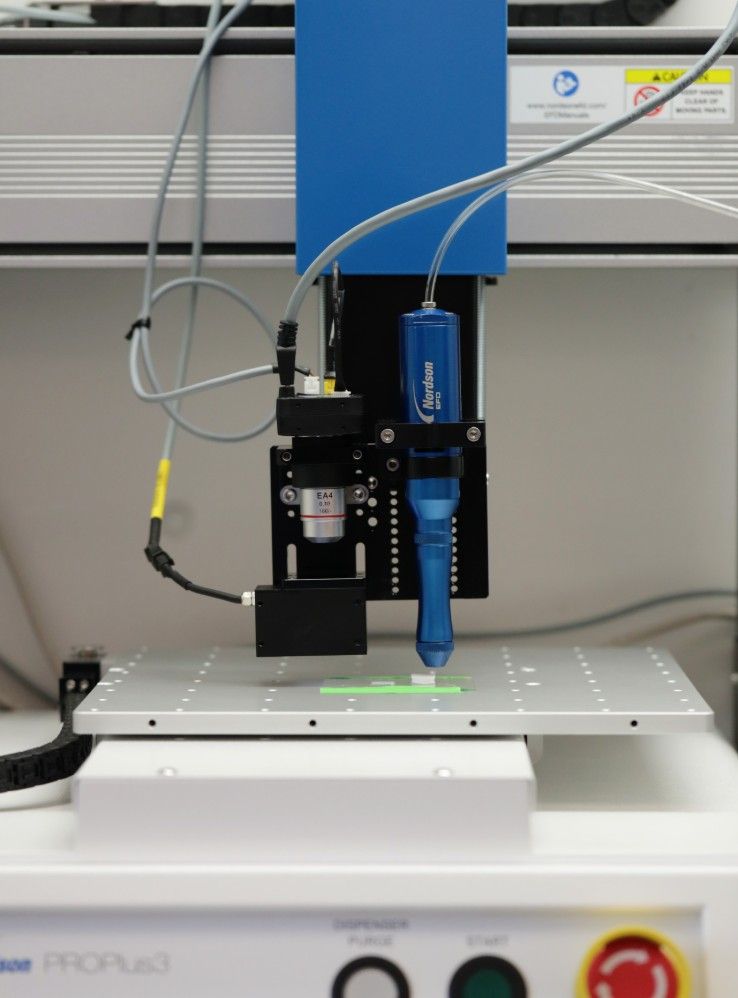
Chow has taken a major step in the field’s efforts to address such a challenge. She and her team in The Chow Lab at Lehigh have demonstrated a new method to fabricate scaffolds presenting spatially organized cues to control cell behavior locally within one material. Their proof-of-concept paper was recently published in Biomaterials Science and is based on work led by graduate students Paula Camacho (bioengineering) and Hafiz Busari (materials science and engineering), with co-authors Kelly Seims (materials science and engineering), Peter Schwarzenberg (mechanical engineering and mechanics), and Hannah L. Dailey, an assistant professor of mechanical engineering and mechanics. Their publication shows how their platform can be used to create continuous, highly organized scaffolds to regenerate two different tissues, such as those found in the osteochondral interface.

Chow’s lab creates biomaterial scaffolds made of biodegradable polymers, which are long chains of molecules that can degrade over time in the body. Scaffolds are widely used in tissue engineering to provide cells with structural support, as well chemical cues that “tell” the cells what type of cell to become or tissue to form. Used in the early stages of tissue regeneration, scaffolds are designed to be implanted in the body and then degrade as new tissue forms.
Chow’s team uses 3D printing technology to control the deposition of “inks” with different material compositions. These inks are prepared by mixing a biodegradable polymer with peptide-modified polymers. The peptides, composed of amino acids, provide the bioactive cues to the cells.
“We know from literature and nature what amino acid sequences we want,” says Chow. “We can take a segment that we know plays a specific and important role in telling cells to grow new tissue and, in a sense, steal from nature. We take a peptide and attach it onto a polymer and add that in while we are constructing our scaffolds. We use 3D printing as a way to control the organization of these peptide-functionalized polymers as well as the scaffold’s architecture.”
Once the team fabricates the scaffold, they “seed” them with cells, such as human mesenchymal stem cells that can be “coaxed” in response to the peptides into becoming different cell types.
As Chow explains, changing the scaffold’s properties is simply a matter of changing the inks loaded in the printer. The team can modify peptide concentration as well as location, and they can do this with more than one ink composition.
“What we are doing is creating an environment that fosters the regeneration of two different tissues simultaneously in one scaffold,” says Chow. “We make a scaffold that has the correct cues―one that promotes cartilage, one that promotes bone―all in one material. You then have a single scaffold where you don’t have to worry about mechanical failure at the interface because you have a single material rather than “gluing” two separate scaffolds together and just hoping for the best.”
Read the full story in the Lehigh University News Center.
Story by Lori Friedman