
By Marc Deluca and Jeff Fetterman
Making Medications Safer with Systems Engineering
All approved medications have been demonstrated to benefit certain populations of patients when used appropriately. All medications may also present risks to patients, especially when used inappropriately or when used for the wrong patients. Regulators such as FDA and EMA require pharmaceutical companies to implement routine risk minimization activities for patient safety as a stipulation of their approval of any medication. Routine risk minimization activities may include documenting the benefit-risk profile of the medication in Prescribing Information, providing specialized patient information in the form of a Medication Guide, and communicating marketing messages in a way that is compliant with regulatory standards.
In certain medications, regulators judge the risks of the medication to be too great in comparison to its benefits. In these cases, special enhanced risk minimization may be required. In the US, the FDA defines this type of risk minimization program as Risk Evaluation and Mitigation Strategies (REMS). Elements of REMS may include special communications to healthcare providers or patients known as a Communication Plan, or it may include Elements to Assure Safe Use (ETASU), which augment the process of prescribing, dispensing, administering, and monitoring medications to order to improve the benefit-risk profile. ETASUs can include healthcare provider training and certification, patient enrollment, and evidence of safe use conditions such as lab testing.
A systematic and rational approach to the design of risk minimization activities would include analytics about the performance of the process, optimization of the process, and ongoing improvement. Unfortunately current FDA and EMA regulations do not specify how to develop effective risk minimization activities, so prevailing approaches tend to be non-systematic and opinion-based.
Jeff Fetterman, a Lehigh alumnus and currently a member of the Lehigh Healthcare Systems Engineering Industry Advisory Council cofounded a company, ParagonRx (now part of inVentiv Health), that uses systems engineering methods to design and implement risk minimization activities in an evidence-based, systematic way that is likely to be more effective and more adaptable for continuous improvement.
Failure Modes and Effects Analysis – A Case Study
ParagonRx provides a broad set of services, but an example of using evidence and systems tools to develop risk minimization activities in an overall risk management program is the use of an adaptation of failure Modes and Effects Analysis (FMEA) to assess the risks in the human-based process of medication management (DeRosier, Stalhandske, Bagian, & Nudell, 2002). In this approach, the business team maps the process of prescribing, dispensing, administering, and monitoring the medication of interest. A cross-functional team evaluates each step in the process to anticipate where the process may fail to protect the patient from the inherent risks of the medication. After identifying the underlying causes and possible patient effects of those failures, the team scores the frequency and severity of the failure occurrence (i.e., they assign a Hazard Score). The team defines an appropriate risk minimization activity for all failures with a high hazard score. The risk minimizations are included in a risk management plan for implementation and continuous improvement. As an example, a pharmaceutical company had brainstormed a set of 30 possible risk minimization activities based on intuition. Although this is obviously not a scientific approach, it is not uncommon at this time in the industry. In this case, the pharmaceutical company was appropriately unsettled by the lack of rigor in their approach, so they engaged ParagonRx to “validate” their risk minimization design by using FMEA to identify where the medication management process may fail to protect patients and to use the 30 predefined tools as the risk minimization activities wherever possible.
After mapping the process and conducting the cross-functional assessment to identify possible process failures, our team found that only 16 of the 30 predefined risk minimizations were relevant to this risk management program; 14 of the predefined risk minimizations were not usable and were irrelevant to the program. In addition, we identified 12 other (new) tools that had not been previously contemplated, but were necessary to assure a complete risk management program. We consolidated the resulting 28 relevant and new risk minimizations into programs that were distributed across four stakeholders, assuring backup redundancy while alleviating burden on the healthcare provider.
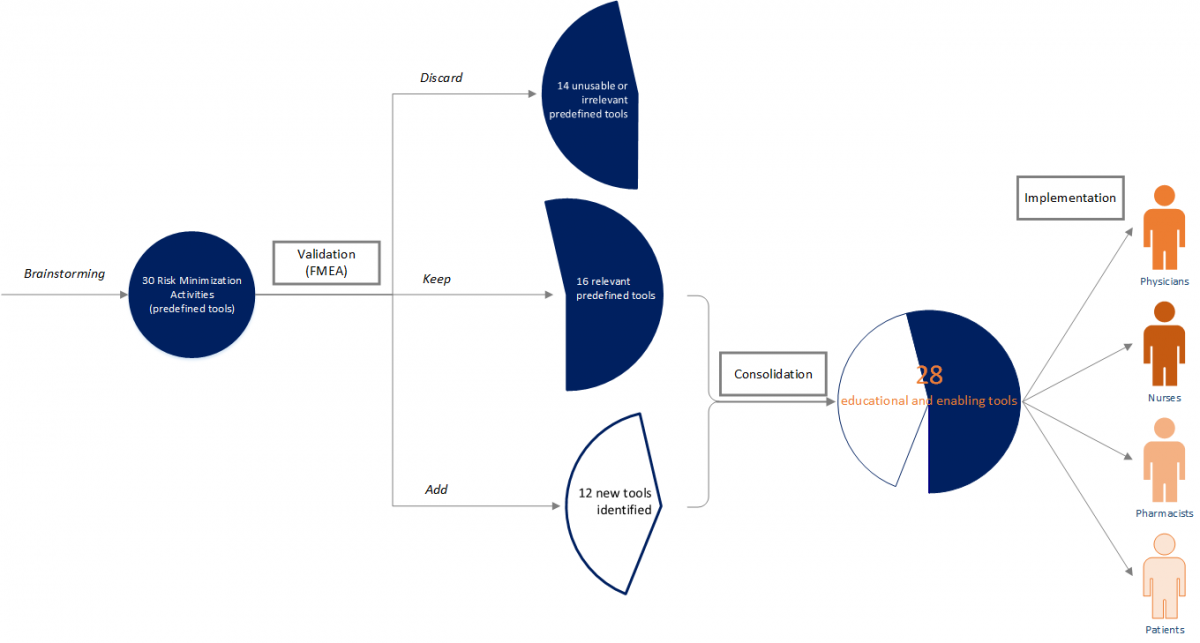
Our experience in this engagement and many others supports two key points: (1) intuitive brainstorming, while common, is highly inaccurate, causes waste and leaves gaps; and (2)the use of systematic approaches, such as the FMEA, creates a more focused risk management program, with fewer and more targeted tools.
Ad-hoc risk management program design can be detrimental to both a company’s bottom line and program stakeholders’ (e.g., prescriber, pharmacists, and patients) experience with the risk management program. Often, programs are developed based on precedents and opinions of regulators and industry experts, sponsors and vendors. Time is money; spending the time to proactively assess a product’s risk related to the care delivery process can delay approval and entry to market. There is a perception that proactive risk management is costly and too difficult to implement rapidly to meet aggressive timelines. However, shortcuts taken at the time of program design can result in programs that do not integrate into our stakeholders (e.g., prescribers and pharmacies) workflow, creating additional burden to their already time constrained days. This burden creates an environment that not only adds complexity to healthcare providers, but also can adversely impact patient access to life saving products. Additionally, not “getting it right” the first time can delay program launch, leading to increased program development and implementation costs needed to modify a program’s design to meet the needs of our stakeholders while maintaining regulatory commitments.
Applying systems engineering methodologies proactively to complex problems can minimize implementation and operational problems that may be encountered during program implementation. We have seen this repeatedly in many industries, and systems engineering methodologies are now finally making their way to pharmaceutical risk management. This approach enhances the development process by applying an evidence-based assessment, which systematically identifies and prioritized potential issues while providing a framework for collecting data to measure program performance. Metrics identified during the risk assessment can be used to trigger corrective actions as part of ongoing continuous quality improvement initiative, closing the gap between when a potential issue is identified and when a corrective action is implemented. While the focus of this article is risk management, these tools are applicable to virtually any process and can realize significant benefits, from reducing waste to improving clarity in communication and expectations, and to optimizing effectiveness and efficiency.
Marc Deluca is an alumnus of Lehigh Healthcare Systems Engineering program. Marc completed the HSE program online, while supporting and managing risk management initiatives at ParagonRx. He is now at TEVA Pharmaceuticals, where he serves as the Senior Manager of Risk Evaluation and Mitigation Strategies (REMS) Programs.
Jeff Fetterman is also an alumnus of Lehigh, but from the Chemical Engineering program. Jeff has founded two companies in the pharma space, one of which is ParagonRx, and is on track of starting a third. He dedicates some of his spare time to serving on the Industry Advisory Council of Lehigh’s HSE program.
