 Quantum dots are nanoscale crystalline particles that act as semiconductors. When exposed to UV light, they emit energy in the form of light, and the size of the quantum dot determines the color it emits.
Quantum dots are nanoscale crystalline particles that act as semiconductors. When exposed to UV light, they emit energy in the form of light, and the size of the quantum dot determines the color it emits.
“So by tuning the particle’s size, you can essentially tune its optical properties,” says Mark Snyder, an associate professor of chemical and biomolecular engineering (ChBE), “which makes them interesting materials to use in applications like bioimaging.”
The problem, however, is that quantum dots are typically synthesized using toxic solvents, high temperatures, and hot injection methods. “All of which create challenges from the standpoint of sustainability and scalability,” says Snyder.
To overcome those challenges, researchers seek to create high-quality, nontoxic, functional quantum dots via green, scalable synthesis routes.
Snyder and his team recently published a paper in the Journal of Materials Chemistry B that outlines just such a synthesis method. The project was funded by the NSF’s Scalable Nanomanufacturing program, and was a collaborative effort among labs led by Snyder, ChBE department chair Steven McIntosh, and Harold B. Chambers Senior Professor in Materials Science and Engineering Christopher Kiely. The lead author is Nur Ozdemir ’22 PhD (pictured), a recently graduated member of Snyder’s lab.
 Earlier work by McIntosh, Kiely, and the University of Virginia’s Bryan Berger had explored a biomineralization-based approach to synthesize quantum dots using low temperatures and aqueous conditions. Biomineralization is the process by which biological systems produce inorganic materials.
Earlier work by McIntosh, Kiely, and the University of Virginia’s Bryan Berger had explored a biomineralization-based approach to synthesize quantum dots using low temperatures and aqueous conditions. Biomineralization is the process by which biological systems produce inorganic materials.
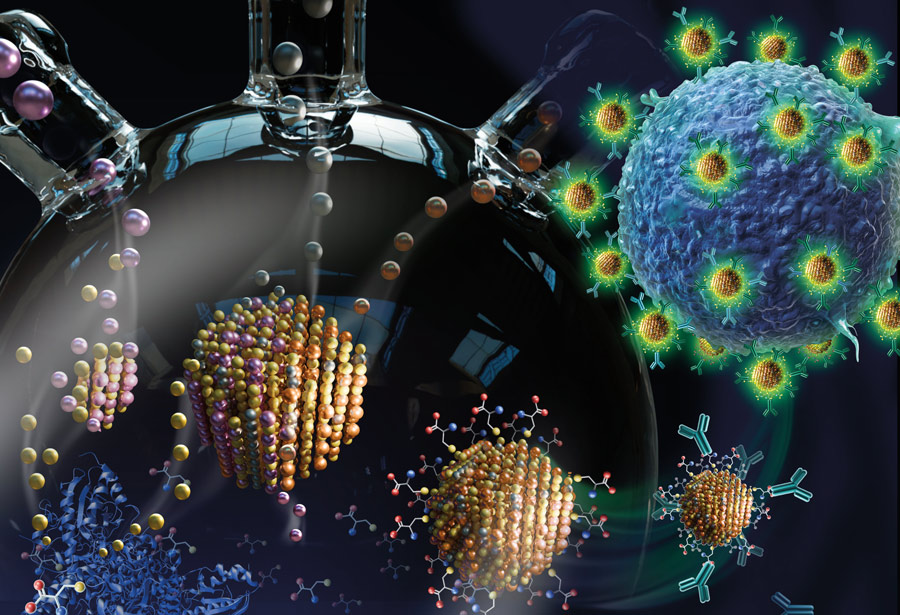
“The question that Nur was addressing with this paper was how to use biomineralization to synthesize quantum dots with greener and tunable compositions,” says Snyder. “She was able to show that she could do this in ‘one pot,’ meaning, she could grow high-quality particles with tailored composition in a single vessel. And under these aqueous conditions, the particles themselves are hydrophilic, so they can be used directly in aqueous applications without any complex purification steps. And they’re also easily functionalized—in this particular case, with antibodies that were specific to certain cellular systems.”
For this application, Ozdemir collaborated with co-author Shannon Collins ’21 PhD, who at the time was researching antibiotic resistance with Lehigh ChBE associate professor Angela Brown.
“I think this paper is an excellent example of student-driven research,” says Brown, who is also one of the paper’s authors. “I think it was over lunch one day that Nur was talking to Shannon about wanting to show that these quantum dots could be used for targeted imaging, and Shannon was able to help her do that.”
Brown says that, together, the students were able to show proof of concept. The quantum dots, functionalized with an antibody, could successfully target receptors for that antibody on specific cells.
“What’s so interesting about the quantum dots is that they can be made to emit different colors,” she says. “And so, in theory, you could look at multiple things on a cell, such as the presence or absence of a specific protein associated with disease or the distance between two different proteins to provide information about how those proteins interact with each other. So there’s a lot of possibilities there in terms of identifying what’s going on with the cell—is it healthy, is it dying, is it changing in some way?”
The synthesis process, too, opens a number of doors.
“Even though we looked at silver indium sulfide with zinc incorporation, the approach should be applicable to a whole range of other compositions in the metal sulfide class of materials,” says Snyder. “A broader materials palette could potentially now be accessible because of the work that’s been done here to unravel some of the complexities in the biomineralization system.”
Illustration (above) by SayoStudio, featured on the cover of the June 28, 2022, issue of Journal of Materials Chemistry B.
