 In a pharmaceutical approach to disease treatment, blood transports drugs throughout the entire body, sometimes producing undesirable side effects. Electroceutical or bioelectronic therapy offers a more selectively targeted approach, leveraging the electrical impulses that regulate the body’s functions through the nervous system, with the goal of affecting only the organ of focus.
In a pharmaceutical approach to disease treatment, blood transports drugs throughout the entire body, sometimes producing undesirable side effects. Electroceutical or bioelectronic therapy offers a more selectively targeted approach, leveraging the electrical impulses that regulate the body’s functions through the nervous system, with the goal of affecting only the organ of focus.
Electroceuticals are not new. The pacemaker, which sends electrical signals to stabilize heart rhythms, was invented in the 1950s. However, nerve stimulation is not yet broadly approved to treat other diseases. Vagus nerve stimulation devices, for instance, have only been approved by the FDA to treat epilepsy and depression.
Mayuresh Kothare (pictured), the R. L. McCann Professor of Chemical and Biomolecular Engineering, and his colleagues are investigating how the neurostimulation of peripheral nerves—the nerves located near various organs—could be used to treat other diseases, just as pacemakers are commonly used to help regulate heart function. With approximately $2.2 million from the National Institutes of Health (NIH) through the Simulating Peripheral Activity to Relieve Conditions (SPARC) program, the team is developing software and modeling tools for optimizing the delivery of neurostimulation signals to peripheral nerves to treat conditions such as cardiac arrhythmia, hypertension, and stomach and bladder disorders. Little research exists on the use of electroceuticals to treat such disorders.
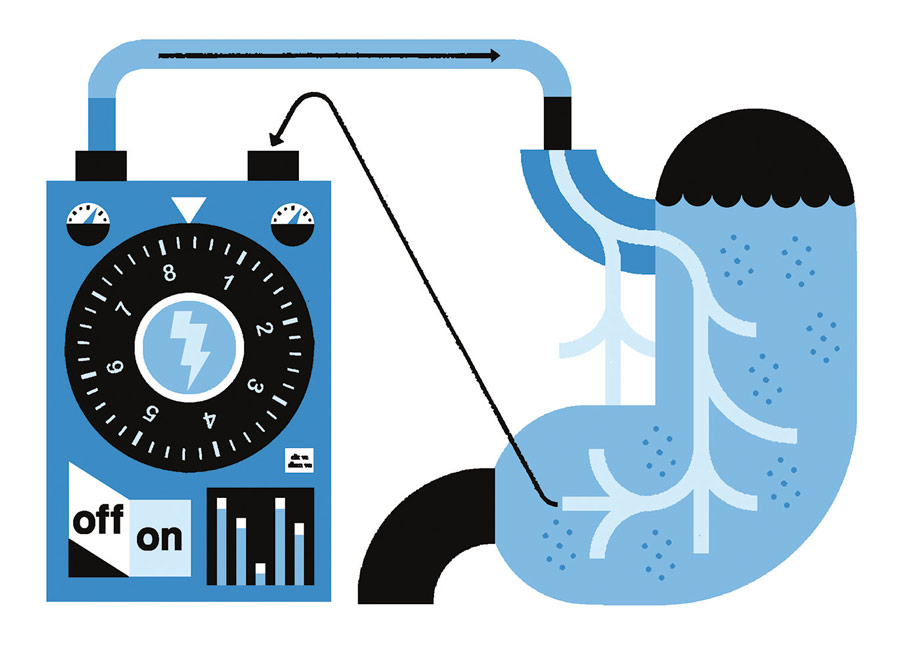 “Nerves connect different organs,” Kothare explains. “There is a nerve connecting to the heart, there is a nerve connecting to the stomach, and there is a nerve connecting to the bladder. So those nerves are the ones that control the function of each organ. We are looking at typical diseases that affect [the heart and stomach]. We are trying to develop techniques, combining engineering and experimental approaches, that would allow us to treat those diseases through neural stimulation.”
“Nerves connect different organs,” Kothare explains. “There is a nerve connecting to the heart, there is a nerve connecting to the stomach, and there is a nerve connecting to the bladder. So those nerves are the ones that control the function of each organ. We are looking at typical diseases that affect [the heart and stomach]. We are trying to develop techniques, combining engineering and experimental approaches, that would allow us to treat those diseases through neural stimulation.”
The team is working to determine how to generate the correct response to effectively treat the right problem, Kothare says. In the case of a pacemaker, wires are implanted in the heart and connected to an implanted chip so the pace of the heartbeat can be monitored and adjusted, if necessary, through the delivery of electrical energy. Similarly, Kothare says, common disorders of the stomach, such as constipation or irregular rhythms of the stomach that cause chronic indigestion, could theoretically be treated by stimulating the gastric nerve, thereby changing the contractions of the muscles of the stomach.
“The grand challenge is how do you design these electrical signals so that they do what you want them to do?” he says. “Because if you send the wrong neural signal, it could create a worse symptom. Just like with drugs—a drug can have a good effect or a bad effect.”
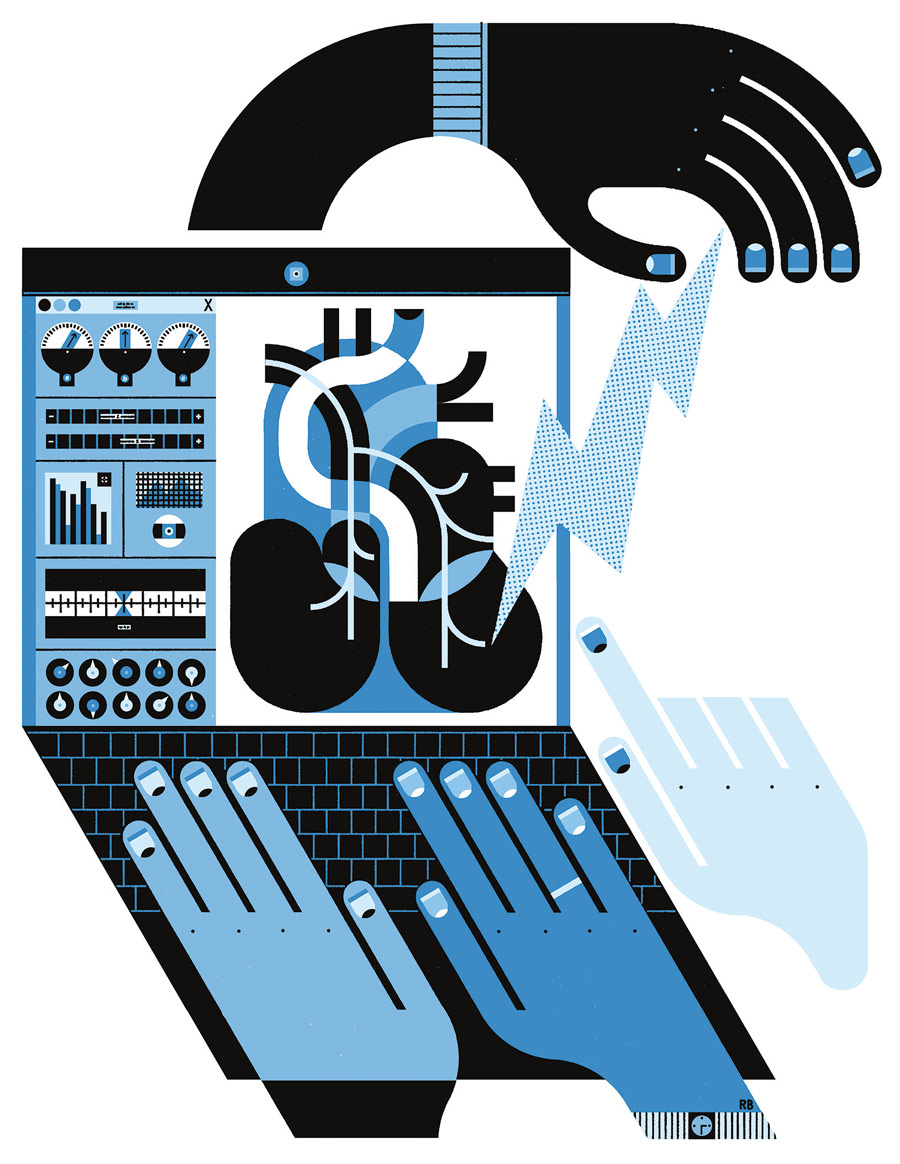 Kothare and Babak Mahmoudi, a machine learning scholar from Emory University, lead the project. Collaborators include Charles Horn, a neuroscientist from the University of Pittsburgh; Raj Vadigepalli, a professor of pathology, cell biology, and anatomy from Thomas Jefferson University; and Gautam Kumar ’08G ’13 PhD, an assistant professor of chemical and materials engineering at San Jose State University. Mark Arnold, a retired Lehigh computer science faculty member, helped develop novel approaches to software and cloud-based simulations for the project. Students and postdocs from across the institutions are also collaborating on the work.
Kothare and Babak Mahmoudi, a machine learning scholar from Emory University, lead the project. Collaborators include Charles Horn, a neuroscientist from the University of Pittsburgh; Raj Vadigepalli, a professor of pathology, cell biology, and anatomy from Thomas Jefferson University; and Gautam Kumar ’08G ’13 PhD, an assistant professor of chemical and materials engineering at San Jose State University. Mark Arnold, a retired Lehigh computer science faculty member, helped develop novel approaches to software and cloud-based simulations for the project. Students and postdocs from across the institutions are also collaborating on the work.
The group is using a two-pronged approach. Horn is collecting experimental data by implanting electrodes in the gastrointestinal system of ferrets to stimulate the gastric nervous systems. Team members are also analyzing existing data, such as cardiac rhythm data collected from dogs by UCLA, which is where the modeling, machine learning, and cloud computing pieces come in.
“What we are trying to do is to develop mathematical models of the behavior of the organ in response to different stimuli, and then use these mathematical models to develop the best or ‘optimal’ signal that could eliminate the disorder,” Kothare explains. “So that requires us to have an understanding of the underlying biophysics. How do the muscles contract? How does the contraction of the muscle move the liquid inside the stomach? What prevents the liquid from flowing out of the stomach continuously? These are the kinds of quantitative, almost engineering-like questions we are asking to understand in the form of equations and models—how we can predict the behavior of organs in response to stimulation. When we don’t fully understand the underlying biophysics, we employ data-driven machine learning models to capture the observed behaviors.”
This story was adapted from the Lehigh Research Review (2022 Volume No. 7). Illustrations by Raymond Biesinger
