Himanshu Jain, director of NSF’s International Materials Institute for New Functionality in Glass, was searching for a biologist to help him understand how a biocompatible glass interacts with human cells.
Matthias Falk, assistant professor of biological sciences, was looking for a materials scientist to help develop a new biocompatible material for orthopedic surgeons.
Both work at Lehigh but it was on a flight to Japan that their paths first crossed. Falk, who uses fluorescence light microscopy to study the gap junctions through which cells communicate, was traveling to Sapporo to make a presentation at the 16th International Microscopy Congress. Jain, an expert in glasses, was on his way to Kyoto University to give the keynote talk at the Second International Symposium on New Materials Science.
The transcontinental meeting initiated a partnership that combines cell biology and materials science to seek new ways of repairing bone that has been broken in accidents or ravaged by disease.
Doctors have found grafting, or implanting natural bone, preferably from the patient, to be one of the most effective ways to rebuild strong bone. But grafts sometimes require additional surgery and extensive remodeling and cannot always be used.
So researchers have started testing bioactive glass as a “scaffold” to help the body rebuild its natural bone. Recent observations show that chemicals leaching from the glass actually help grow bone on the scaffold.
With funding from NSF, Jain and his team have for several years been designing bioactive glass scaffolds that are biomechanically similar to the host tissue and encourage tissue growth.
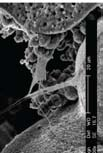
The team, which includes dental researchers in Egypt and materials scientists in Portugal, places healthy progenitor cells on the scaffold, which is located in the injured or diseased area. To optimize bone regrowth, they use a scaffold that blends macropores (100 microns or wider to allow bone cells to grow inside the glass) with nanopores (up to 20 nanometers in diameter to promote cell adhesion). The team has developed two novel techniques for fabricating the dually porous scaffolds.
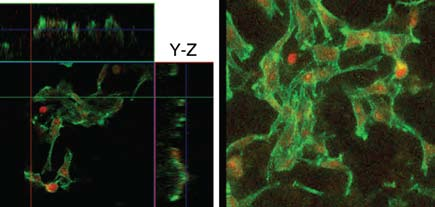
To verify that the scaffolds are biocompatible and that they promote bone cell adhesion, Jain’s team has used scanning electron microscopy (SEM) and fluorescence light microscopy.
The latter technique enables Falk and his students to quickly detect and quantify the number of cells that adhere to the scaffold and to observe their shape, viability and skeletal organization. Falk says a large body of literature supports the conclusion that bone-precursor cells require direct, gap-junctionmediated, cell-cell communication during development and for differentiation.
The samples made so far have been mechanically weak, and questions remain about how fast and fully the scaffolds will dissolve in the body. With funding from the Howard Hughes Medical Institute, Jain and Falk are exploring these and other questions in vitro. Their goal is to create the most effective bone scaffold possible for in vivo testing by Jain’s partners in Egypt.
Falk is a welcome addition to the project, says Jain, because he is investigating how to make biocompatible glass that is not only not toxic to the body but actually helps prevent infection, and because he is studying how the glass can facilitate the development of precursor cells into mature bone cells.
Falk is also observing the chemicals that leach from the glass and induce the differentiation of precursor bone cells. Working with Jutta Marzillier of Lehigh’s Genomics Facility, he is using Quantitative Real-Time Polymerase Chain Reaction to determine how certain genes are switched on so that the precursor cell becomes the final bone cell.
“Thousands of people have benefited from bone implants,” says Jain, “and we expect the demand to grow as our population ages. Many of today’s implants, made with metal, do not last long enough, often cause pain and sometimes require subsequent operations.
“What Matthias and I are hoping to do is identify those glass scaffolds that are the most biocompatible and bioactive, so that the bone regenerates and the glass poses no threat to the body and eventually fully dissolves. The idea is to help the body grow its own parts. So far, the nano-plus-macroporous scaffolds are the most promising.”