The far-flung stardust and common matter that constitutes our planet and the world around us, bears within still, after millennia of human inquiry, countless riddles yet to be figured despite the most sophisticated tools we can devise being brought to bear against them. Solving the most urgent problems facing society in many cases will depend on finding new ways to crack the secrets of these stubborn substances.
That is the inspiration for the launch of the new Institute for Functional Materials & Devices (I-FMD), one of three new Interdisciplinary Research Institutes (IRIs) that create communities of scholars and catalyze crucial research in areas where Lehigh is poised to make lasting societal contributions.
As with the recently inaugurated Institutes for Data, Intelligent Systems, and Computation research institute (I-DISC), and for Cyber Physical Infrastructure and Energy (I-CPIE), I-FMD will be a crucible for new ideas at the crossroads of disciplines.

In areas like material fabrication and synthesis, and with materials such as semiconductors, composites and polymers, researchers are working across traditional boundaries to overcome interrelated challenges inherent in the development of tomorrow’s energy systems, health advances, and a broad array of other fields.
“Research into new and existing materials, and the processes by which they are created, improved, refined, and utilized, is a longtime core strength across Lehigh’s engineering community,” says Himanshu Jain (photo, left), director of I-FMD and Lehigh’s Diamond Distinguished Chair and Professor of Materials Science and Engineering. “Over time, we have leveraged the University’s history of industrial collaboration in this space into a world-class research engine that cuts across many disciplines. Through the I-FMD, we are looking to build a more structured community of scholars focused around these topics. It’s a grassroots effort to engage all of the relevant perspectives from around campus, and around the world.”
The aim is to get collaboration right, says Steven McIntosh (photo, right), the Class of 1961 Professor of Chemical and Biomolecular Engineering associate director of I-FMD.
“We want to get our faculty-led research teams out of ‘stovepipes’ and into projects where they can work on bigger problems,” he says. “Society’s challenges require a wide array of skills and perspectives; the idea is to break paradigms and change the culture; Lehigh’s new Institutes support and celebrate interdisciplinary awareness and thinking across our research community.”
That is the essence and mandate of I-FMD, to think broadly and pragmatically about discoveries that can make a difference. With materials, innovation is a multi-step process that begins with inventing new materials or improving existing ones, finding ways to produce them efficiently and economically, manipulating the materials to suit the needs of the new devices and applications, and finally, developing ways to create the materials at scale.
“This is the nature of the problem we face now, especially for what we want to do with materials all the way to devices,” says McIntosh. “Individual research cannot conceptualize the material, make the new material, understand how it will function in a device, put all the components of a device together and make it into a feasible system or application. That takes a lot of different expertise. We have that expertise on campus and in our network of collaborative institutions and partners, so we have to aggressively work across boundaries and go after these things.”
The I-FMD institute is broken down into five areas of emphasis: biomedical functionality, chemical functionality, mechanical functionality, optical and electrical functionality, and processing and manufacturing. The participants in I-FMD include more than 80 faculty members spread across 10 departments, so creating opportunities for dialogue is key.
“If we want to go after grand challenges, that means looking at problems in a holistic way, and dealing with the full complexity of the issues, and not just fix one part of it,” Jain says. “A good materials person can create a better tire. If you want to improve transportation systems, you have to pull together a lot of experts in pursuit of the same goal. We will facilitate this in part with regular workshops that help connect our members to external partners and each other, where we can have intelligent conversations about technical aspects of our work, and do this across colleges and departments. We are also making a special effort to include graduate students and other members of our research community.”
Even where researchers have competencies in similar niches, something as rudimentary as having different vocabularies for similar concepts can be a stumbling block.
“We want in these conversations to find common interests and strengths,” says McIntosh. “Ideally, people come in and have a freewheeling talk, identify problems and limitations, and drill down on them together.”
Bloody brilliant
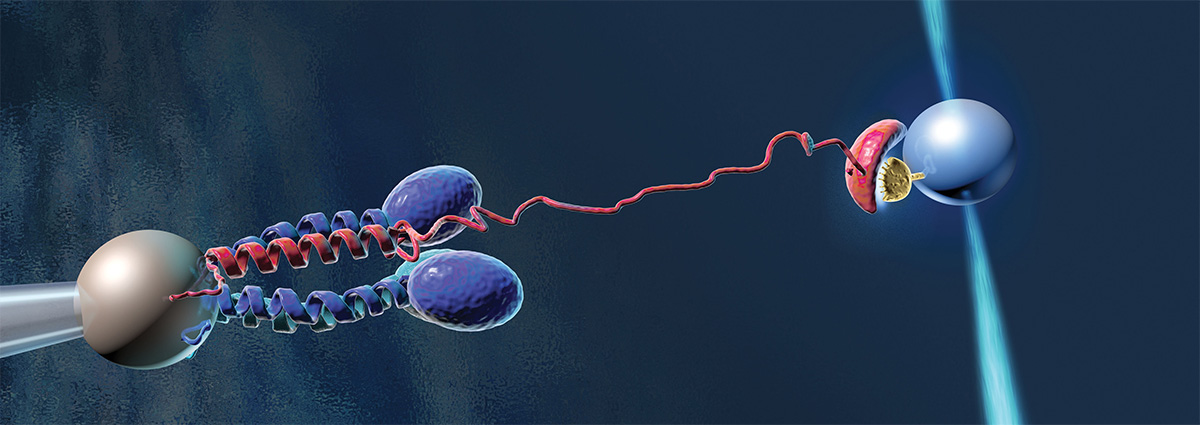
As part of Lehigh’s expanding health science and medical device initiatives, a team of I-FMD researchers is looking at a vital and complex clotting agent in blood, von Willebrand Factor (VWF). VWF is a plasma protein that helps clot blood in the areas of cut blood vessels. When blood flow increases, VWF senses the change and opens up to capture platelets and help them adhere to the walls of blood vessels, enhancing clotting. In essence, VWF jump-starts the clotting process when regular clotting cannot occur due to blood flow conditions. VWF is noteworthy in that it is one of the few compounds in the body that has a mechanical response—it is the physical stress of the increasing blood flow, which activates the unfurling of VWF.
Xuanhong Cheng, associate professor of materials science; Alparslan Oztekin, professor of mechanical engineering; Edmund Webb, associate professor of mechanical engineering; and Xiaohui (Frank) Zhang, associate professor of bioengineering, are bringing a formidable array of tools to learn more about the enigmatic compound. Zhang, who specializes in biomechanics, and Cheng, whose expertise is in microfluidics, are in charge of the experimentation and observational side of the project. Webb and Oztekin are handling the modeling and computation.
“Our model has a level of detail other authors don’t have,” says Webb. “When you marry that to our experimental data it is really powerful.”
Learning more about VWF could lead to the alleviation of thrombosis, which can result from unwanted quantities of activated VWF in bloodstream. Maladies from dysfunctional VWF affect up to 3 percent of the global population. In addition, there is significant research on medical devices that shows that devices put into the body can alter blood flow conditions and activate VWF. A better understanding of VWF could help doctors counteract that. In addition, there are therapeutic possibilities for VWF-like compounds, which would employ the trigger mechanism in VWF for as-needed drug delivery in the body.
In its collapsed state, VWF circulates freely in the bloodstream. When activated, it can elongate to 10 times its original size. “The molecule has many sites for interacting and forming bonds of collagen and platelets that get exposed when it opens,” says Webb. “Also, when it is recirculated into the blood, there is an area of VWF that, when it is exposed, you get a fairly quick reaction to cut the VWF at that site so you don’t have large molecule floating around the bloodstream. It’s really a fascinating entity.”
The team is combining single-molecule force spectroscopy and microfluidic imaging to track VWF in varying flow environments. “We have set up a microfluidic channel, and tagged the VWF molecules fluorescently to see how they respond to differing rates of flow,” Cheng says. Oztekin and Webb are doing coarse grain modeling, using a base chain model made up of two beads and one string to represent a monomeric unit in VWF.
“The model is unique, and we also have the advantage of the experimental results to sharpen the parameters for the model,” Oztekin says.
A section of the molecule is of particular note with regard to targeted drug therapies, Webb says, and its dual function makes it especially intriguing.
“It is a domain in VWF called A2. It’s a nonlinear elastic spring. When it is partially opened it can react with platelets, but when it is fully opened it exposes a site that will cut the VWF down to size.”
One aspiration for the team is to find ways to synthesize a compound that would mimic the properties of the A2 domain, to similarly respond to a flow change in the bloodstream and release a drug payload. “The idea is that if there is a normal flow happening in circulation, in a place where plaque is built up or place of internal bleeding there would be abnormal shear stress.” Cheng explains. “We could take advantage of the molecule’s mechanical response to different flow patterns to deliver drugs to dissolve plaque or coagulate molecules to stop internal bleeding. Once we understand the mechanisms of how A2 changes conformation under flow, we can make molecules rather than rely on A2, as it turns out that VWF is not easy to make.”
In the team’s sights is a polymeric design that mimics the critical functions of VWF.
“The basic biological properties of VWF have been elucidated, though as yet there is more to learn about the detailed biomechanical properties,” says Zhang. “That said, our study has already revealed significant insights that would lead to the design of synthetic molecules based on VWF.”
 Taking aim at silicon’s inefficiencies
Taking aim at silicon’s inefficiencies
Siddha Pimputkar, an assistant professor of materials science and engineering, has focused recently on the production of gallium nitride crystals, which are difficult to produce in quantities with the level of quality technical applications require, and demand high temperatures and pressures in the fabrication process.
One of the major uses for gallium nitride, a wide bandgap semiconductor, is power electronics. “Think of electric cars, where you have a huge battery, and the voltage needs to be altered to run the engine,” says Pimputkar. “Today’s technology is built on silicon, which is not super efficient. You can lose up to 10 percent of the energy to heat. We can do better.”
Another major market is LEDs, as the crystals can yield bright blue emitting LEDs, which has enabled blue lasers and energy-efficient lighting technologies.
Pimputkar is developing improved ways to create gallium nitride crystals at scale that meet the demands for size and quality. One method is an ammonothermal technique that dissolves gallium into a supercritical ammonia solution, and precipitates gallium nitride onto an existing seed crystal.
“We put the crystal we want to grow in a hot temperature zone of the autoclave, add high purity gallium nitride and create a temperature gradient between the two,” Pimputkar explains. “This arrangement leads to a natural cycle of fluid motion due to the density differential between the two zones, ultimately causing gallium nitride to be transported from the source to the seed and precipitate out. The challenge is how to find ways to growth the crystal faster and ensure uniformity within and across all growing crystal boules.”
With fellow I-FMD members Kai Landskron, associate professor of chemistry in the College of Arts and Sciences, and Nicholas Strandwitz, assistant professor of materials science and engineering, Pimputkar is at work growing other binary and ternary nitrides, including indium gallium nitride, which requires developing a novel growth process.
“Indium is a big atom and when added to a gallium nitride crystal reduces its bandgap leading to longer wavelength emissions in LEDs--effectively going from blue to green to red light. As we squeeze more of them into a gallium nitride crystal, the atomic spacing expands, and you eventually get a large mismatch to the gallium nitride substrate,” Pimputkar explains. “This adds more strain and limits how thick a layer you can grow before introducing dislocations to relax that strain. If the strain hits a critical point, the efficiency of the device will drop off substantially. Starting with an indium gallium nitride crystal as a substrate possessing a larger atomic spacing would alleviate this problem, though such a crystal hasn’t been demonstrated yet.”
The menu of nitride crystals Pimputkar is growing make up a range of variable bandgap semiconductors that can be leveraged for uses in solar cells, sensors and other expanded uses. In August of 2018, he and Landskron were awarded a $450,000 grant from the NSF to explore the growth of cubic boron nitride using more scalable, sustainable methods.
“There is a lot of interest in substances that are tunable, both from an atomic spacing aspect, where you can change lattice parameters or atom spacing to fit the device you want to grow on top of it, and from an electronic and chemical properties perspective,” Pimputkar says.
 A quantum leap for quantum dots
A quantum leap for quantum dots
I-FMD associate director Steve McIntosh is also part of a research team fashioning “quantum dots” for serious industrial application. These are nanocrystalline semiconductor compounds used in applications such as lasers, transistors and medical imaging, and currently manufactured using expensive techniques that yield toxic byproducts.
McIntosh, along with Christopher J. Kiely, the Harold B. Chambers Senior Professor of Materials Science and Engineering and Bryan Berger of the University of Virginia (formerly of Lehigh), through an “Emerging Frontiers in Research and Innovation” award from the NSF, had previously identified biosynthetic, environmentally sustainable routes toward the creation of quantum dots, a process which could bring about positive change across a number of industries.
The team, which has grown to include Mark Snyder, associate professor of chemical and biomolecular engineering, has recently received a four-year, $1.5 million award from the NSF to focus on scalable manufacturing of nanocrystalline oxides and their structuring.
The inspiration for the new process came about while Berger was working with the Lehigh Valley Health Network to find ways to combat a drug-resistant microbe, Stenotrophomonas maltophilia. Berger noticed that when fed silver, a common antibacterial agent, it induced the microbe’s threat response system to encase the metal in a kind of plaque and conveniently spit it out in the form of quantum dots.
The original group teamed up to see where the discovery would lead, beginning their exploration with selenides and sulfides, then, with the help of a Lehigh accelerator grant, shifting their focus to working with oxides. They also replaced the microbe with silicatein and other enzymes. The group is collaborating with the Cardiff Catalysis Institute in Wales, where researchers will test the team’s oxide-based end products to see how they perform as catalyst materials.
One of the major innovations of the team’s way of producing selenide and sulfide-based quantum dots is that it is done at room temperature and in aqueous solution, making it far more environmentally friendly than current industrial techniques. Since the precursor materials are virtually benign, there is no toxic waste from the manufacture to dispose of, and since high temperatures and pressures are not required, the process is more energy efficient. The team has also been working on producing cerium oxide, and has now further expanded this to ceria zirconium oxide. One major use for these latter materials is as catalytic supports.
The ramifications of the team’s work could be vast. For example, the global market for automotive catalytic converters alone is forecast to reach $273 billion by 2024, and there is potential for other industrial and consumer applications as well.
“The challenge is to bring the quantum dot manufacturing to a scale that makes it industrially viable, and we are still very much in the infancy of that process,” says Kiely. “We want to take it to the next level and investigate what other kinds of oxides we can generate by biomineralization, and see if we can grow them directly onto a pre-formed support.”
That goes to Snyder’s specialty.
In the quantum dots, you have a scale of around 10 to 100 atoms,” explains Snyder. “Until now, the focus has been on these nanocrystalline samples. I have been using technologies in my lab to structure oxides through particle assembly or templating; we are looking to apply these concepts to bridge toward larger scale structured materials.
“At the larger scales,” he continues, “challenges include supporting nanocrystalline particles in a controlled way on an inert or even structured support, creating higher order structures comprised of these small nanocrystalline oxides, or fashioning nanocrystalline materials into porous structures where molecules can easily transport to and from the surface to carry out a reaction.”
The addition of zirconium increases the catalytic performance and stability compared to cerium oxide and creates a better catalytic support, says Kiely.
“The challenge is controlling how much zirconium we can dope into the cerium oxide. Can we controllably insert 10, 20 or 40 percent zirconium? We’ve had some success on this front, but need further development to more closely control the composition of the material once we add in the new component.”
According to Snyder, the funding that Lehigh has provided to this research is a promising model for innovative projects.
“The early seed project led to an initial grant, and now a larger grant,” he says. “Our work has brought together faculty that have different but complementary skill sets and interests, and it’s the kind of approach that we hope can continue to grow across the university.”
Kiely concurs. “I think that is part of the philosophy of these Institutes, to create more of these groupings and allow us to make more connections to industry and other external partners. and find new ways to exploit these materials,” he says. “This quantum-dot project falls squarely under the founding idea of I-FMD, and the materials we are producing actually have a chemical function as optoelectronic materials and catalysts. It all fits beautifully.”