|
Additive manufacturing coordinator Trevor Verdonik, donning a full suit of protective gear as a precaution against spreading germs, works on face shield components in Wilbur Powerhouse. |
Lehigh’s campus should have been buzzing with post-spring-break energy. Instead there was silence. Even research activities, the steady current that drives the university forward, had been forced to power down.
At least on the surface.
In the early days of the COVID-19 crisis—and continuing today—Lehigh engineers found creative ways to contribute to the community response by applying and advancing fundamental knowledge within their disciplines. They answered the call to help hospitals weather a shortage of personal protective equipment (PPE) and are exploring new ways to make disinfectants more effective. They’re innovating as they pursue a better understanding of the virus and vaccines and other methods to curb its spread. They’re even digging into the social ramifications of “going remote.”
Uncertain. Unprecedented. Unique. The pandemic has proven to be all of these things. At the same time, there is reassuring familiarity in the roll-up-the-sleeves, problem-solving approach that engineers in the Rossin College are taking to rise to our present challenge.
|
Courtesy of St. Luke’s University Health Network |
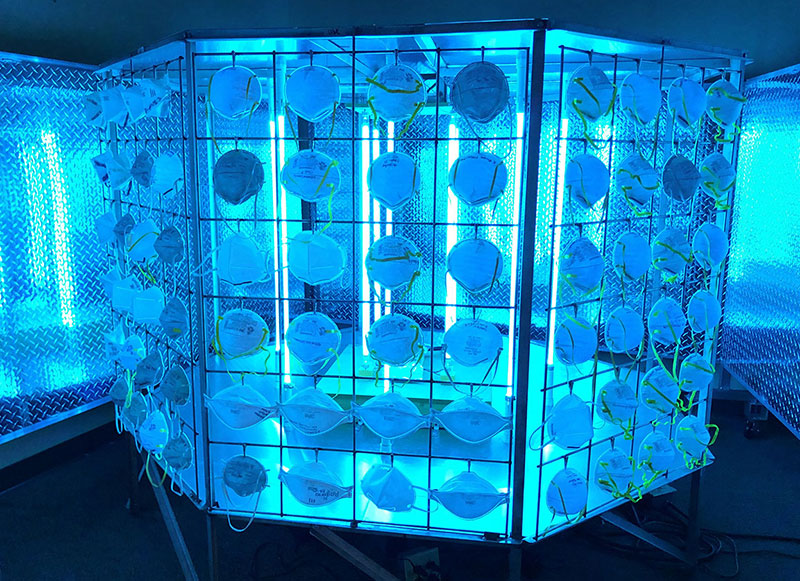
‘Bug Zapper’ invention safely sterilizes N95 masks
When the request from Dr. Christopher Roscher, an anesthesiologist at St. Luke’s University Health Network, hit Nelson Tansu’s inbox in mid-March, his response was swift:
Yes, most likely, it could be done.
Faced with the nationwide shortage of N95 masks, St. Luke’s knew it needed a safe, effective way to extend its existing supply for medical professionals treating COVID-19 patients. Roscher’s personal research exploring the use of UV light for PPE decontamination in a pandemic situation led him to Tansu, the Daniel E. ’39 and Patricia M. Smith Endowed Chair Professor in the Department of Electrical and Computer Engineering (ECE).
“We approached this idea understanding that in an ideal world we would have a new mask for everybody who needed one,” says Roscher, “but the reality of the situation is that we need to conserve.”
Hours after the initial email, Tansu and his colleagues discussed a potential plan with Roscher. The next day, he gathered an enthusiastic team of volunteers—staff and students from Lehigh’s Center for Photonics and Nanoelectronics (CPN), where Tansu serves as director, and the ECE department, all practicing social distancing in their homes.
“If we are on board, then we can transform this idea into reality,” Tansu told them. “Together with the team of doctors, we have to figure out a solution that we can build in our garages.”
And that’s what they did. Collaborating via Zoom meetings, phone calls, and hundreds of emails and text messages, Tansu, Roscher, and their team designed, completed the engineering fabrication of, and installed the device in less than three weeks—without ever stepping foot on Lehigh’s campus or meeting face-to-face.
Now in use at St. Luke’s, the “High-Throughput Symmetrical and Non-Shadowing Ultraviolet Sterilization System” (nicknamed the “Bug Zapper” because of its resemblance to the insect-killing backyard device) exposes the masks to UV-C light. This specific range of ultraviolet light can cause changes in the DNA and RNA of viruses and other pathogens, including novel coronavirus, effectively deactivating them. The team has filed two patent applications associated with the invention.
The system has a large, octagonal metal frame with UV lights positioned at its center to achieve symmetrical UV-C irradiation on the N95 masks. Its targeted capacity is approximately 3000 N95 masks per day (200 per exposure), but that figure can scale up to 10,000 masks per day if necessary.
|
Members of the Lehigh–St. Luke’s team, which has applied for two patents, connect via videoconference to discuss the project. Said Nelson Tansu. “This is one of the quickest turnarounds from idea to execution that I have ever experienced." (Photo Courtesy of Nelson Tansu/Used with permission) |
The goal, says Tansu, was to use enough UV-C light to damage viruses and bacteria but retain the integrity of the N95 mask, which can be degraded more significantly over time by steam or chemicals. Staff members at St. Luke’s monitor exposures with a radiometer, which measures the amount of light irradiation to which the masks are exposed.
The final design—a true collaborative effort—was ready in just two days. “Everybody just kept building on the idea,” says Anthony Jeffers, a research engineer in the CPN. “We were on the same level all the way through: We were just there to try and solve the problem.”
To observe distancing guidelines, the team then divided the project into individual tasks that allowed them to build the device modularly from home.
“The first staff member built a certain part and the second staff member built the second part,” explains Tansu. What followed, he jokes, might have seemed suspicious to a casual observer: Each team member dropped a part off in a specified location at a particular time and remained in his or her car to watch over it from afar until the St. Luke’s representative picked it up. Finally, they assembled it “like a LEGO set,” says Tansu.
With the remote assistance of Tansu and his team, the team at St. Luke’s conducted tests to determine the appropriate dose of UV-C light, as well as microbiological tests to determine the device’s effectiveness.
“Designing something, optimizing it, completing further analysis, putting together an experimental plan, creating it, testing it, and using it typically takes a very long time, a period of months, especially in academia,” says Tansu, who is a Fellow of the U.S. National Academy of Inventors. “But this is one of the quickest turnarounds from idea to execution that I have ever experienced. We are very fortunate to have such a committed team in completing this important task in such a short time.”
—Kelly Hochbein
|
Multiple headband iterations were 3D-printed during the prototyping step to optimize the face shield design and the production process. |
Design lab team takes on essential role in 3D printing face shields
I wish there were some way we could help the doctors and nurses in New York.
It was Brian Slocum’s first thought when he read news describing how doctors were treating coronavirus patients without adequate personal protective equipment. He immediately started researching ways to solve the problem.
“And I very quickly found that people all over the world were working on either 3D-printed or custom manufactured solutions,” says Slocum, who is managing director of Lehigh’s Wilbur Powerhouse and Design Labs, and a Lehigh alum himself. “I thought, I wonder if we could do that?”
At nearly the same time, Slocum started receiving emails from local hospital administrators who were worried about their supplies of protective gear. They wanted to know if Lehigh could help them.
Slocum soon found an article published by Prusa Research, a Czech company that had developed a clear plastic face shield that could be 3D-printed, and was open-sourcing their design. Slocum responded to the administrators, telling them Lehigh could absolutely produce something like it.
“It prevents droplets from people coughing or sneezing from hitting the doctor’s face,” says Slocum. “It also increases the life of the N95 masks that providers are wearing.”
Slocum, along with additive manufacturing coordinator Trevor Verdonik ’13 ’15G, a PhD student in materials science, and Michael Moore ’12, assistant manager of the design labs, began iterating on the Prusa design. They were soon joined by the product development team at Knoll, a design firm located 20 miles from campus.
The medical professionals the team consulted with identified a few problems with the Prusa design. A gap between the forehead and the plastic face shield could potentially allow droplets in from above, and the elastic band that went around the head couldn’t be cleaned properly.
To address the first problem, the Lehigh and Knoll teams designed a 3D-printed dual headband. One band rests directly against the forehead, and one sweeps out in front to hold the face shield. They designed pegs along both bands, which allowed them to secure a neoprene comfort guard that acted as both a cushion for the forehead band and a roof that closed the gap between the two bands. An adjustable, easily sanitized neoprene head strap solved the second issue.
Lehigh designated Slocum, Verdonik, and Moore as essential workers, which allowed them to work on campus. Verdonik operated the 3D printing of the headband and the support pieces that attach to the bottom of the shield for better structure, while Moore used the laser printers on Mountaintop Campus to cut the shields (from PET plastic), the neoprene comfort guard, and the neoprene strap. (Listen to episode 4 of the Rossin Connection podcast as Slocum’s team takes you inside their labs to explain their production process.)
|
Nurses at Lehigh Valley Health Network are among the health care providers who have received face shields manufactured at Lehigh. (Photo courtesy of Lehigh Valley Health Network) |
By early May, they had delivered more than 1200 shields to local hospitals and emergency management agencies. At that time, DuPont had donated a 1000-foot roll of PET plastic, enough for 1000 shields, and the team had set up a crowdfunding site to ensure they were able to continue meeting local needs. They hit the 5000 mark at the end of June, as mass manufacturing began to catch up with demand. “We were able to help fill the gap,” says Slocum.
The team’s ability to finalize a design so quickly was due in large part to their ability to leverage a variety of technologies—including 30 or so 3D printers that had been sitting idle with students no longer on campus—and get the best out of each.
“We know how to use these tools, and we’re good at designing solutions,” says Slocum. “So we have the capabilities from the physical resources, but also on the intellectual side.”
Their efficient response also reflects the connections the university has with the community.
“We’ve spent a long time and a lot of institutional resources building relationships with our local hospitals,” says Slocum. “Pretty much everybody I’ve dealt with both at St. Luke’s and Lehigh Valley Health Network at some point has toured the additive lab, and I’ve talked to them about how additive manufacturing can be brought to bear on some of their health care needs. Leveraging those resources and relationships allows us to be proactive community partners.”
Slocum, Verdonik, and Moore never anticipated becoming essential workers during a pandemic. But when the call from those in need came, there was never a question how they—or the university—would respond.
“People have been so appreciative,” Slocum says. “And it just feels so good to do something where you’re helping somebody stay safe during this chaotic time. That, to me, is the core of what we should be doing as humans, as engineers, as Lehigh practitioners. That’s where we can make the greatest difference in the world.”
—Christine Fennessy
|
A conceptual illustration shows the SARS-CoV-2 virus binding to an ACE-2 receptor on a human cell. (Credit: Kateryna Kon/Shutterstock) |
Understanding the mechanism of infection
The world is anxiously awaiting a vaccine to curtail the spread of COVID-19 and bring the pandemic to an end.
By imitating a virus, a vaccine triggers the body’s immune system to generate a series of responses, including the production of antibodies by B-lymphocytes. If the body is then exposed to the actual virus, these antibodies will recognize it and neutralize it.
But a vaccine may not be a panacea for this crisis.
“How long that immune response will last is a big question,” says Frank Zhang, an associate professor of bioengineering and mechanical engineering and mechanics. “And the virus could mutate. Right now, the mutation rate for SARS-CoV-2 [the virus that causes COVID-19] is not that bad. But in a few years, it could mutate to the point where the vaccine doesn’t work anymore. Think about our experience with the flu vaccine. Every year, the CDC has to predict how the flu will mutate, and design the vaccine accordingly. Sometimes the vaccine works well, and sometimes it doesn’t. So it’s much better to have antiviral drugs that don’t rely on a human immune response.”
The development of such drugs is Zhang’s ultimate goal. He and his team are using atomic force microscopy (AFM) and optical tweezers (a tightly focused laser beam that can isolate and move micron-scale objects) to study how a surface protein called a spike protein on the SARS-CoV-2 virus interacts with human cell surface receptors.
“One known receptor is the ACE-2 receptor, which is expressed in many cells, but specifically in epithelial cells in our upper and lower airway,” says Zhang. “That’s why when the coronavirus is inhaled, it attaches to those epithelial cells. The spike protein not only sticks to the ACE-2 receptor, but it also has some built-in activation and invasion steps that we still don’t understand. But essentially, the virus finds a way to get into those epithelial cells and replicate itself, and that’s how we get infected.”
If researchers better understood that three-step mechanism of infection (attachment, activation, and invasion), they could potentially develop antiviral strategies to block one or more of the steps and keep the virus from entering human cells in the first place.
To maximize their efficacy, however, Zhang says those antivirals should target well-conserved processes for viral entry, meaning those mechanisms that essentially don’t change, no matter how much the virus itself mutates. For example, the attachment step is not well conserved: Currently, the virus attaches to the ACE-2 receptor. But it could mutate, and adhere to a different receptor. Conversely, the invasion step—when the virus essentially punches a hole in the human cell, inserts its genome, and starts replicating—is very well conserved, says Zhang.
“No matter how the virus mutates, that mechanism is not going to change much,” he explains.
While significant research is being conducted on this invasion step, little is known about the activation step. By using AFM and the optical tweezers, and working in tandem with faculty members Wonpil Im, a molecular modeler, and Anand Jagota, an expert in biomechanics, Zhang is developing experimental techniques to understand how the spike protein changes shape and gets activated.
—CF