Professors Muhannad Suleiman, Bryan Berger, and Derick Brown (clockwise from left) recently received an 18-month grant from the National Science Foundation to explore silicatein’s potential as a soil strengthening agent. (Photo by John Kish / Special to Lehigh University)
A sea sponge enzyme could provide greener, firmer soil foundations.
Liquefaction is hardly a household word, but the phenomenon is all too familiar to the residents of Christchurch, New Zealand.
Between 2010 and 2016, Christchurch experienced five major earthquakes. The largest in magnitude struck in February 2011, killed 185 people and destroyed much of the city’s infrastructure. It was the third-worst natural disaster in New Zealand’s history.
Much of the destruction was the result of liquefaction, which occurs when water saturates loose or sandy soil and breaks the bonds between sand grains or soil particles. Under pressure such as that from an earthquake, soil behaves like a liquid and loses its ability to support structures. Liquefaction can also cause soil to shift or slide down even a gentle slope.
During the February 2011 earthquake and its aftershocks, wrote The Press, Christchurch’s largest newspaper, liquefaction became the “scourge of Christchurch,” causing thousands of tons of grey sandy silt to bubble out of the ground.
The Christchurch example, and the damage from liquefaction during the 1964 earthquakes in Japan and Alaska, says Muhannad Suleiman, shows that a structure is only as strong as the soil on which it stands. A bridge or skyscraper can be built with the best materials and construction techniques, but it can't withstand earthquakes or other natural hazards if it rests on unreliable soil.
Suleiman, an associate professor of geotechnical engineering, leads an interdisciplinary team of researchers who are enlisting the aid of an enzyme found in deep sea sponges to provide buildings, bridges and other structures with a firmer, more environmentally friendly soil foundation.
The enzyme, called silicatein, has shown promise in precipitating the formation of flexible calcite (calcium carbonate) in soils. The calcite acts as a cementing agent and strengthens soil by causing its tiny particles to bond together.
Suleiman and two other associate professors, Bryan Berger of chemical and biomolecular engineering and Derick Brown of civil and environmental engineering, have received a grant from the National Science Foundation to synthesize silicatein in the lab and explore its potential as a soil-strengthening agent. The group also has a Faculty Innovation Grant from Lehigh. They believe they are the first group to test the enzyme for this application.
Silicatein shapes up
Engineers have traditionally strengthened weak or loose soil by compacting it or by grouting it with cement, says Suleiman. This helps soil resist the loading imposed by natural hazards. But these two traditional methods are energy-intensive, and the production of cement also generates significant carbon dioxide.
More recently, Suleiman and other researchers have had success using the microbes, or bacteria, that occur naturally in soil to precipitate calcium carbonate (one of its forms of precipitation is calcite.) The process is called microbial induced carbonate precipitation, or MICP; Suleiman is collaborating with a biologist in Qatar to use MICP to improve soil’s performance under sand storm conditions. But MICP has limitations. It yields a brittle calcite bond and also produces ammonia, a hazardous material. And the microbes, which measure 1 to 2 microns across, are too large to penetrate the tiny pores that are typical of clayey and silty soils.
These drawbacks, says Suleiman, limit the types of soil to which MICP can be applied and the ability of soil to withstand large strains such as those generated during earthquakes. They also limit the potential for MICP to be used in supporting structural foundations and in mitigating liquefaction.
By contrast, says Suleiman, silicatein promises to produce a stronger, more flexible bonding of soil particles. Because the enzyme is much smaller than a microbe, it can be potentially used in much finer soils. And silicatein has two additional assets.
“Sustainability and environmental friendliness are the main advantages of our method,” says Suleiman. “We’re working with a natural material.”
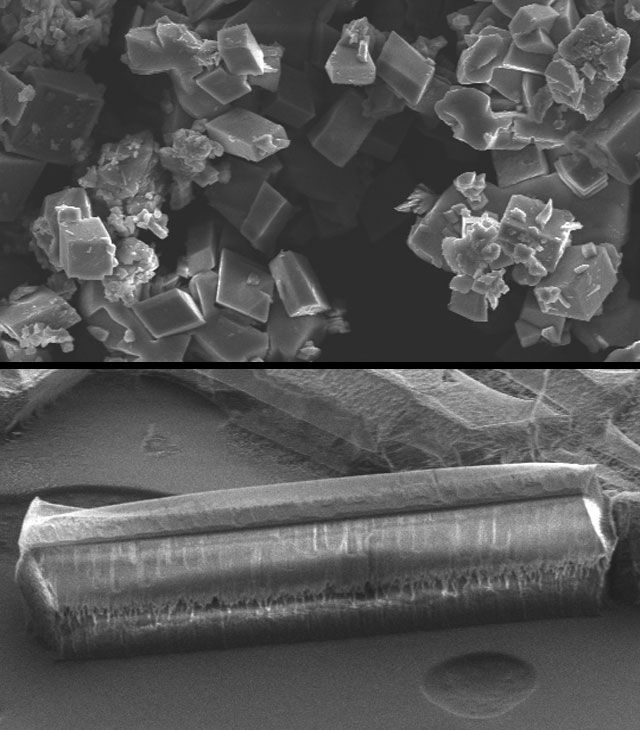
One key to silicatein’s success, says Suleiman, is that it precipitates the formation of rod- or needle-shaped calcite crystals. Their elongated structures enable the crystals to form stronger and more flexible bonds between particles of soil.
“Based on research by our group and others, silicatein causes a specific formation of calcium carbonate. Instead of precipitating calcite crystals that are cubic in shape, silicatein changes the crystallization into a needle shape.”
The typically cube-shaped calcite crystals, says Suleiman, “produce brittle bonds that break very quickly under stress. By contrast, calcite precipitated with silicatein, because of its rod-shaped crystal, forms strong, flexible bonds that can take a lot of stress without breaking.”
Less cost, more precision
To achieve the optimal shape of the calcite crystals, Berger modified an existing method of synthesizing the silicatein enzyme.
“We read reports from other researchers that said the enzyme could be added to a solution of calcium to template the growth of needle-shaped crystals. What we do is to make a high-concentration solution of the chemicals that form calcite. Then, when the solution begins to crystallize, we add the enzyme.
“The enzyme causes the calcite to crystallize into the structure and shape that we want, which the calcium solution will not do naturally on its own.”
Suleiman decided to test silicatein’s soil-strengthening potential in 2015, when he and his group came across an article published by researchers from Johannes Gutenberg University and the Max Planck Institute in Germany. Writing in Science, the group reported the creation of a new synthetic material made of silicatein and calcite, characterized by a “rubber-like flexibility” and inspired by the spicule, a structural element of the sea sponge.
Suleiman spoke with Brown after reading the Science article. Brown urged him to contact Berger, who was working on a project that employs silicatein to synthesize the metal oxide nanoparticles that are used in catalysts, separations and other applications.
This came as a welcome surprise to Suleiman.
“I honestly did not even know that somebody else at Lehigh was working with this enzyme,” he says.
Berger and three other faculty members — Steve McIntosh and Mark Snyder of chemical and biomolecular engineering and Chris Kiely of materials science and engineering—have an Accelerator Grant from Lehigh to scale up the biomanufacturing of functional, nanostructured materials.
The current methods of synthesizing many metal oxides, says Berger, require heat, high pressure and chemical solvents that are expensive and toxic. Silicatein overcomes these drawbacks.
“The skeleton of the sponge that produces silicatein is composed of mineralized silica. The sponge utilizes the silicatein enzyme to mineralize sand to form its skeleton. So it has a natural ability to convert metal to metal oxides. We modify the silicatein to mineralize metal precursors into crystalline metal oxides at room temperature and pressure and using only water.
“It’s a much cheaper and more environmentally friendly method than the current chemical methods.”
Silicatein also makes it possible to synthesize nanoparticles and other products with greater precision than conventional chemical methods, says Berger.
“Chemical synthesis is homogeneous. Combined with the high temperature and pressure that are required to convert metals into metal oxides, this makes it difficult to control the synthesis and direct the oxide nanoparticles where you want them to be. With our method we can control the deposition of the enzyme and more easily fabricate materials.”
Fate and transport
Working with McIntosh, Snyder and Kiely, Berger has achieved some success in scaling up the production of silicatein, which has benefited his work with Suleiman and Brown.
“Without the ability to make large quantities of the enzyme, it’s difficult to translate this into a useful technology for industries,” says Berger. “I learned how to do this through a trial-and-process while I was working with Steve, Mark and Chris, and it was a contribution that we were able to make early to Muhannad and Derick’s project.”
Suleiman, Berger and Brown are also attempting to engineer silicatein so it provides the optimal bond properties between soil particles, enabling the soil to remain stable when subjected to natural hazards.
“We have looked at different percentages of silicatein and how they affect the shape and the form of the crystals,” says Suleiman. “Based on preliminary results, we have found that, generally, 5 to 10 percent of silicatein gives us the optimal crystal shape. Once we go beyond that percentage, we don’t see a lot of these rod-shaped precipitations.”
This spring, the researchers plan to test the silicatein-precipitated calcite crystals in soil.
“Because it’s an enzyme, we need to look at the sorption of silicatein onto the sand and ultimately onto soil,” says Brown, whose expertise lies in soil chemistry and in the fate and transport of bacteria through soils. “That will affect how far we can transport it. We are concerned with the transport of the enzyme and of any other constituents. We want to see how far out this cementation would extend.”
The partnership has been an education for Suleiman, Berger and Brown.
“I am not an expert in the geotechnical side or in enzyme production,” says Brown. “And Muhannad’s and Bryan’s backgrounds are not in soil chemistry and sorption at the soil surface as mine is. So we’ve taught each other.”
“It’s been a mutual learning experience for all of us,” says Suleiman. “We meet and try to get to the point where we speak the same language. Similar words could mean different things in different fields. But we now understand roughly what each other is doing.”
“Working with soil and foundation engineers is totally new to me,” says Berger. “It’s been fascinating. Previously, I’ve been able to apply what I do—engineering biological systems—to the solution of medical problems or the manufacturing of materials.
“This latest project allows me to apply my work to environmental problems. I didn’t realize there were so many opportunities.”
Story by Kurt Pfitzer