The fabrication of drug-delivery systems, a multibillion-dollar industry, is faced with two competing challenges: achieving sufficient drug load (DL) in delivery media while avoiding the socalled “burst effect.”
This burst effect – in which a large drug volume is quickly released into the body – can be dangerous. Furthermore, the drugs’ expense makes it advantageous to contain them in the carrier as long as possible and to release them in a sustained manner.
Anthony McHugh, the Ruth H. and Sam Madrid Professor and department chair of chemical engineering, has made significant progress in solving both issues by encapsulating drugs in a honeycomb-like polymer matrix, thus helping attain controlled drug release while providing maximum DL to the delivery system.
“The techniques for making polymeric membranes have been around for a long time,” says McHugh. “What’s new about what we’re doing is how we are applying those principles to judiciously control drug-polymer interactions in delivery systems.”
In previous work, McHugh discovered inadequacies in methods of delivering drugs that were intended to dissolve in polymer solutions and then to re-coagulate into membranes after they are injected into the body. He and his team developed and patented improved drug-delivery technologies, and reported their work in the Journal of Pharmaceutical Research, the Journal of Controlled Release and the Journal of Membrane Science.
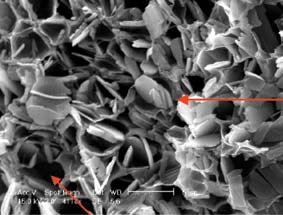
Recently, McHugh has focused on embedding low molecular-weight drugs, such as Naproxen, into polymeric films, either for direct implant in the body or as a coating on stent-like devices. His studies have found that DLs at or below the drug’s solubility limit of 23% behaved much as expected, and triggered the burst effect at DLs between 23% and 35%.
However, with still greater DLs of 40% to 46%, something quite surprising came to light.
“At these high concentrations,” says McHugh, “it seems that the polymer and drug solidify in a way that creates the desirable controlled release. In essence, the polymer’s honeycomb structure actually stabilizes and traps the drug. The results of this study indicate that one can produce polymer structures that allow for higher drug loads and longer sustained release periods, with no burst effect.”
McHugh, who receives funding from NSF, Merck and ALZA Corp., is pursuing these avenues, utilizing biodegradable polymers and microparticles that are more suitable for human or animal implantation.