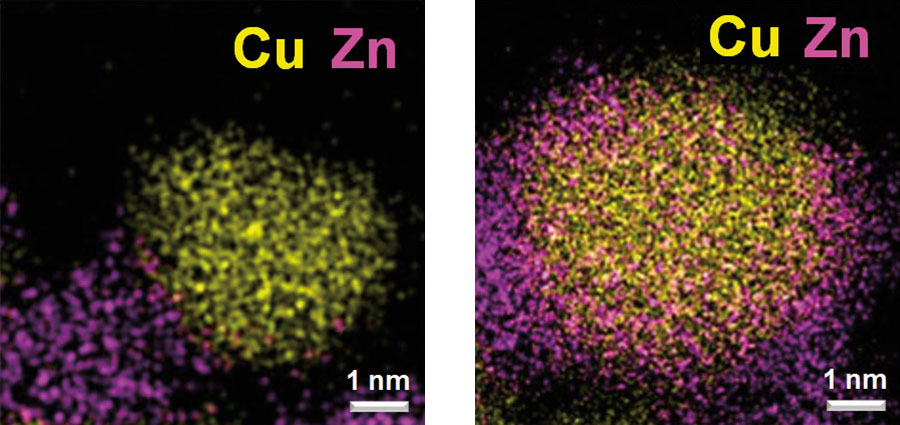
At left, the catalysts are mostly present as separate particles after activation with molecular hydrogen. At right, zinc oxide "decorates" metallic copper particles after induced activation.
The science of catalysis—the acceleration of a chemical reaction—is perhaps not the most recognizable branch of study, but it is absolutely embedded into the fabric of modern society.
The development and production of fuels, chemicals, pharmaceuticals, and other goods depend on catalysis. Catalysis plays a critical role in energy generation and the mitigation of humanity’s impact on the environment, and it is involved in the manufacturing of some 25 percent of all industrial products in the U.S. If a thing is made, worn, lived in, played with, driven upon, or otherwise used by people, catalysis likely plays a fundamental role in its origin story.
Research in the field enables new and improved products and more efficient ways of doing and manufacturing, well, just about everything. But advancement in industrial catalysis can be costly in a macroeconomic sense: Wholesale changes that require a “rip and replace” strategy do not sit well with firms and supply chains that power and provision our global economy.
In a paper published in Nature Catalysis, researchers from Lehigh and colleagues from the East China University of Science and Technology (ECUST) propose a novel method of significantly enhancing the catalytic efficiency of materials already in broad commercial usage, a process they’ve termed “induced activation.”
 The team, supported by the National Natural Science Foundation of China and the U.S. Department of Energy’s Office of Science, includes Israel E. Wachs (pictured), the G. Whitney Snyder Professor of Chemical and Biomolecular Engineering at Lehigh, PhD student Tiancheng Pu, and ECUST professor Minghui Zhu ’16 PhD.
The team, supported by the National Natural Science Foundation of China and the U.S. Department of Energy’s Office of Science, includes Israel E. Wachs (pictured), the G. Whitney Snyder Professor of Chemical and Biomolecular Engineering at Lehigh, PhD student Tiancheng Pu, and ECUST professor Minghui Zhu ’16 PhD.
“The surface structure of heterogeneous catalysts is closely associated with their catalytic performance,” explains Wachs. “Current efforts for structural modification mainly focus on improving catalyst synthesis. Induced activation takes a different approach—manipulating the catalyst surface by controlling the composition of reducing agents at the catalyst activation stage where the catalyst is transformed to its optimum state.”
The team says that using the “tried and true” catalytic material copper/zinc oxide/aluminum oxide (Cu/ZnO/AlO3) enables firms to take advantage of the breakthrough without costly retooling.
“This development effectively doubles the catalytic efficiency of these materials, enhancing their productivity and extending the life of the catalyst,” Wachs continues. “And importantly, induced activation can provide significant benefit to industry without shutting down a chemical plant—or the building of a new and expensive one.”
