
In 2021, the United States government outlined its strategy to achieve net-zero emissions by 2050 to mitigate the effects of climate change. The strategy acknowledged that to reach this goal—and, at the same time, create jobs, improve public health, and stimulate growth—would require action across every sector of the economy.
 The wide-ranging chemical industry, for one, is among the heavyweights.
The wide-ranging chemical industry, for one, is among the heavyweights.
“We’re all working toward solving this climate crisis,” says Srinivas Rangarajan, an associate professor of chemical and biomolecular engineering. “Globally, the chemical industry itself emits 1.9 gigatons of CO₂ every year. As chemical engineers, we want to replace fossil fuels as a feedstock and as an energy source.”
Chemical refineries of the future, says Rangarajan, will rely on alternative carbon and hydrogen feedstocks—waste plastics, waste biomass, CO₂ captured directly from the air or water—to make the products we need. And those plants will be powered by variable renewable energy sources. It will require a fundamental rewriting of the script and a reexamination of how chemical engineering is taught.
“We have to basically reimagine how we make chemicals,” he says.
Such reimagining is in full force across a dizzying array of projects within the Rossin College. There are initiatives focused on using electricity to drive chemical reactions. Fundamental explorations into the chemistry required to split water and make hydrogen. Investigations on how that hydrogen could eventually be stored and transported. There’s research into how new and more benign chemistries and materials can be used to make current molecules, and new methods using machine learning are being developed to control and optimize chemical processes in manufacturing.
The list is seemingly endless, so what follows is just a sample of ongoing work in this arena. And while these projects may be spearheaded by chemical engineers, they are, by necessity, informed by the full range of engineering disciplines.
“Our work is extremely cross-disciplinary,” says Rangarajan. “It cuts across multiple length scales, from understanding how materials are synthesized to how reactions occur on an atomistic scale, to how it all comes together in a reactor and in a process plant, and how that plant interacts with the environment and systems around it. It’s a scope that goes all the way from atoms up to the world as a whole. It touches upon topics ranging from chemistry to materials science to process control, economics, and life cycle. We’re constantly thinking about these connections and how they relate to the bigger picture when it comes to sustainability.”
 The quest for kinder catalysts
The quest for kinder catalysts
When power plants burn fossil fuels at high temperatures, nitrogen and oxygen molecules break apart and then recombine to form a class of compounds called nitrogen oxides, or NOx. These gases are major pollutants and contribute to—among other things—acid rain and global warming.
One way to curb such emissions is with a catalytic converter, similar to what’s used in a vehicle.
“The catalytic converter injects ammonia into the plant’s emissions stream, and the hydrogen in the ammonia reacts with the oxygen in the NOx, and the products are nitrogen and water molecules, which are nontoxic and have no environmental impact,” says Israel E. Wachs, the G. Whitney Snyder Professor of Chemical and Biomolecular Engineering and director of Lehigh’s Operando Molecular Spectroscopy and Catalysis Research Lab.
The process that can convert pollution into benign by-products is called selective catalytic reduction, or SCR. Until now, it has been unclear how this reaction actually occurs, and contradictions have long existed between reaction models within the literature. Wachs and his team used a novel, cutting-edge technology called modulation excitation spectroscopy, or MES, to finally identify the correct pathway. Their results were recently published in Nature Communications.
“Very few people have this capability at the moment,” says Wachs, referring to MES. “It allowed us to monitor weak signals that were not detectable in the past, and revealed the details of how the reaction proceeded.”
The finding is significant because having the right reaction model can indicate how to modify or redesign the catalytic converter for greater efficiency.
Wachs points out that the methodology is general enough so that it can be applied across a range of catalytic reactions, including those emitting NOx from automobiles, ships, tractors, and even riding lawn mowers.
“The products that catalysts manufacture represent 20 to 30 percent of the American economy,” says Wachs. “They’re used to make fuel, chemicals, fertilizers, and even pharmaceuticals. Having the hard data that shows the correct reaction mechanism means we now have the potential to positively impact thousands of catalytic reactions.”
MES will next be used in a collaboration between Wachs and Rangarajan that was recently funded by the National Science Foundation.
The project will use MES to determine how certain promising catalysts can convert biomass or CO₂-derived alcohols, such as methanol and ethanol, into acrolein. Acrolein is an important ingredient for making acrylic acid and acrylate polymers, chemicals used in a range of products from adhesives to paints and plastics.
“The chemistry on this catalyst is quite complicated and the structure of the catalyst during the reaction is different from when it’s synthesized,” says Rangarajan. “MES provides unique insights into the dynamics of these structural changes and, in addition, allows us to more accurately calculate how fast reactions occur, meaning the kinetics of these reactions, and if there are short-lived intermediates that are otherwise missed in traditional approaches.”
However, he says, analyzing MES data to get information about reaction speeds and intermediate steps has never been done before. So the team will employ data science and machine learning techniques to process and extract the information.
The team includes Raimun Horn at the Technical University of Hamburg in Germany, who will use a reaction setup called the Compact Profile Reactor (CPR) that will explore how the catalyst and the composition of reactants and products change as they move through the reactor.
“The goal is to find alternate routes to current chemical products and polymer feedstocks from greener, renewable carbon sources,” says Rangarajan. “Currently, acrylic acid is made from hydrocarbons derived from crude oil. Our quest for greener chemistries produced using benign catalysts is important to move to a more sustainable future for manufacturing.”
 A potential power shift
A potential power shift
Thermocatalysis is a process that accelerates chemical reactions by combining thermal energy and a catalyst, which lowers the activation energy required, making reactions more efficient and often feasible at lower temperatures. It’s widely used in petroleum refining, chemical manufacturing, polymer production, the pharmaceutical industry, and environmental applications like catalytic converters. The thermal energy powering these reactions, however, is often generated by burning fossil fuels.
Electrocatalysis, by contrast, can use energy sourced from renewable technologies such as solar, wind, and hydropower. It can also often operate at lower temperatures and pressures, which can enhance safety and reduce operational costs. But despite these advantages, its widespread adoption has been limited, in part, due to concerns about the longevity and reliability of electrocatalytic systems.
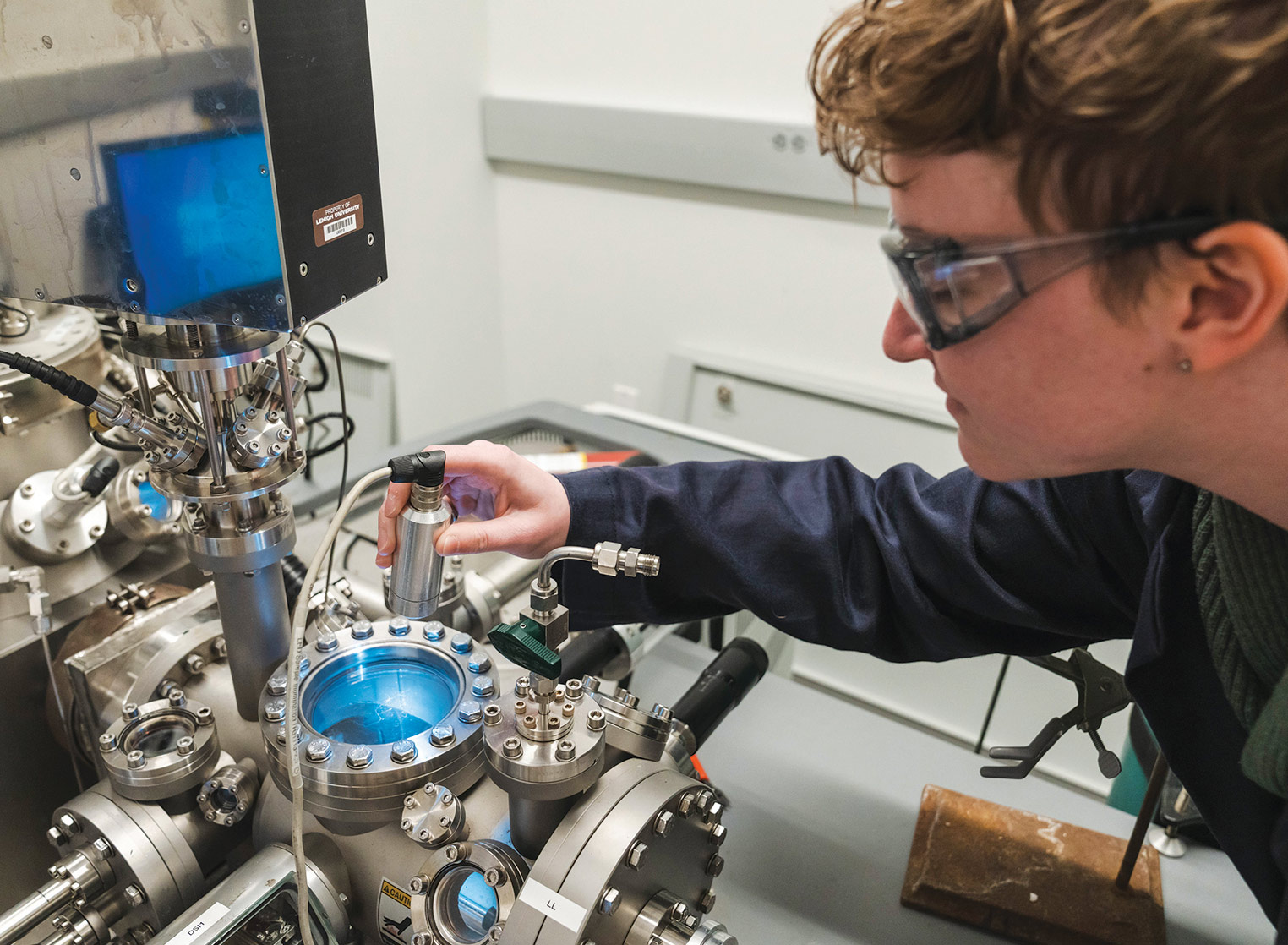
“The goal is to eventually transition industry to electrocatalysis, but to do that, we’re using electrochemical approaches to better understand thermocatalysis,” says Bohyeon Kim (pictured, right), a PhD candidate in the Department of Chemical and Biomolecular Engineering (ChBE).
By understanding the electrochemical aspects of thermochemical systems, thermocatalytic activity can be significantly enhanced through innovative catalyst design. However, current electrochemical techniques often fail to accurately reflect catalytic trends, especially when the adsorption of one reactant is a limiting factor.
Kim, who is part of a team led by Steven McIntosh, Zisman Family Professor of Chemical and Biomolecular Engineering and chair of the ChBE department, is the lead author of a recent paper in the expanding field of using electrochemical approaches to interpret thermocatalytic reactions.
Working in McIntosh’s lab collaboratively with researchers from Cardiff University in the United Kingdom, Kim proposed a novel electrochemical approach that more accurately captures thermocatalytic reactions and the enhancement effects in physically separated bimetallic catalysts. This new method offers a more precise understanding of these reactions, suggesting the way for the development of more effective thermocatalysts.
“If we can make thermocatalysis more efficient, we can take that knowledge and apply it to the design of an electrochemical reactor and make a profound impact on industry emissions,” says Kim.
 Data-driven manufacturing
Data-driven manufacturing
Addressing sustainability challenges is sometimes less a matter of creating or tweaking chemistries or materials, and more one of optimizing and accurately controlling the operation of a process.
“In the old world, if you wanted to assess the changes made to a manufacturing process, you conducted laboratory experiments,” says Mayuresh Kothare, the R. L. McCann Professor of Chemical and Biomolecular Engineering. “And eventually, you move the process from lab scale to manufacturing scale. But in today’s world, with data and machine learning, we can develop models that take the place of those experiments—effectively conducting digital experiments. And when iterated with data from physical experiments to recursively refine the digital model, we end up with a loop that is commonly referred to today as the ‘digital twin.’”
Kothare and Rangarajan are co-leading a Lehigh team that’s working with Owens Corning, a leading building materials manufacturer, in building better models (digital twin) to evaluate the effect of changes to their insulation manufacturing process—changes that could make their process more efficient and accelerate progress toward the company’s sustainability goals.
The project was initiated in collaboration with Lehigh chemical engineering alumnus Dr. Jim Beilstein ’90, vice president for global operations and supply chain at Owens Corning, a member of the Rossin College Dean’s Advisory Council, and chair of the chemical engineering advisory council at Lehigh. The project team includes Sulman Haque, technical program manager at Owens Corning; Lehigh graduate student Siddharth Prabhu; and an extended team of Owens Corning engineers that meet on a weekly basis to chart out research steps.
“In full-scale industrial processes, it is often difficult to measure in real time the effect of process changes on the quality of product,” says Kothare. “Manufacturers usually resort to offline testing to see if products meet specifications. If the product doesn’t meet stringent requirements, it becomes unsellable to their customers.” In an ideal world, manufacturers would have a measurement that shows right away that something is wrong so they can correct the process immediately. The challenge for us has been to create a model and intervention strategy that catches quality problems rapidly enough to ensure that product stays within spec.”
The team is testing a range of new modeling paradigms, specifically physics-informed and interpretable machine learning models. Machine learning is often referred to as a black box of sorts; it provides an output, but no sense of how it arrived at that output.
“So we’re blending interpretable machine learning models with a physics-based set of equations that describe the insulation manufacturing process,” says Kothare. “And then we try to develop a functional relationship for that unknown portion of the model from data-driven techniques. We’d like to be able to give an engineer working on the system a model that can say something like, ‘A 10 percent change in X variable will give you a six percent change in the output.’”
Ultimately, the team’s goal is twofold—to develop software that allows users to change parameters and see how that affects plant performance, and to use that same tool to identify the operating conditions that minimize energy use.
“With the help of Lehigh and the extended Owens Corning team, we are creating new capabilities that drive a deeper understanding of our process, helping us move toward our performance and sustainability goals, and further strengthening the industry-academia partnership,” say Beilstein and Haque.
 A focus on fertilizer efficiency
A focus on fertilizer efficiency
The most commonly used fertilizers around the world contain nitrogen. In 2021, global consumption of nitrogenous fertilizers was more than 195 million metric tons, up from 46 million in 1965, according to Statista.
Nitrogen is an essential nutrient for plants: It enables the growth of robust stalks, flowers, and foliage. However, it’s a difficult nutrient to process. In its free form, nitrogen is inert, and to make it available to plants, it must be chemically processed.
“About five percent of the total global production of natural gas and between one and two percent of global electricity production is used to make nitrogenous fertilizers,” says Jonas Baltrusaitis, an associate professor of chemical and biomolecular engineering. “It’s a very, very energy intensive process, and it’s also critical to sustain a growing population.”
The problem, he says, is that once it’s put into the ground, nitrogenous fertilizer becomes reactive and unstable and only a small portion of it is absorbed by the plants. The rest becomes what’s known as nonpoint source pollution.
“We put all this energy into making fertilizer, just to have half the nitrogen evaporate into the air where it volatizes as ammonia and nitrogen oxides, both of which are greenhouse gases that contribute to particulate matter pollution,” says Baltrusaitis. “The rest of the nitrogen becomes nitrate, which is water soluble and pollutes waterways. So nitrogen is a necessary but problematic nutrient.”
 To solve such problems, Baltrusaitis and his team have developed a chemical process where they co-crystallize urea—one of the most commonly used nitrogen fertilizers. They combine urea, which has low stability in the natural environment and easily hydrolyzes, with various widely available and highly stable minerals (like gypsum), and in the process, create a third material that is far more stable than the parent urea.
To solve such problems, Baltrusaitis and his team have developed a chemical process where they co-crystallize urea—one of the most commonly used nitrogen fertilizers. They combine urea, which has low stability in the natural environment and easily hydrolyzes, with various widely available and highly stable minerals (like gypsum), and in the process, create a third material that is far more stable than the parent urea.
“We end up with a material that contains properties of both parent compounds,” he says. “It has nitrogen, but the minerals provide stability so it decomposes more slowly and becomes less soluble and reactive with water. The fertilizer can then stay in the soil for a longer period of time, which increases its availability for the plants.”
The researchers have scaled the method to the level required by industry, and Lehigh has patented both the process and the materials developed in Baltrusaitis’s lab. The next steps are testing the material in the field to assess the life-cycle impacts of co-crystallization, and working with industry to commercialize the process. The impact of that commercialization could be huge.
“We believe we can revolutionize how these fertilizers are made, how they’re handled, and what their impact is—globally—on food production, the environment, and the economy,” says Baltrusaitis. “We’re talking about transforming an industry that hasn’t changed much in 100 years, making it more sustainable, more efficient, and more economical. The potential for that level of societal impact is extremely exciting, and a huge motivator for us.”
Formula for a greener future
Trying to solve the myriad issues required to hit net zero emissions is a daunting endeavor, to put it mildly. But it’s also a unique opportunity to prepare the next generation of engineers for a differently powered world.
“These are extremely challenging problems, and they’re completely different from what we’ve long been taught in chemical engineering,” says Rangarajan. “When I was an undergraduate, we would learn about crude-oil-driven petrochemical production. But now we’re talking about how to use electricity or waste carbon to drive chemical reactions. We’re talking about developing new chemistries, new catalysts, new materials, and new algorithms. These projects require us to conceptualize the chemical industry of the future, and that’s going to help us better develop the workforce of the future.”



