 Recycling for stronger materials
Recycling for stronger materials
The machining processes of metallic materials such as aluminum create tons of solid waste in the form of very small metal chips. Plus, aluminum scrap parts are usually cut down to small pieces prior to recycling. The combined surface area of millions of tiny pieces creates an extremely high percentage of oxide that renders the material too difficult to melt in the traditional recycling process. Researchers in Germany have developed a solid state recycling technology that involves pressing machined aluminum chips together as a billet and then applying severe plastic deformation to break the oxide into pieces, which allows it to mix homogeneously with the metallic material. Mixing superstrong oxide particles into the aluminum matrix makes the material stronger than regular aluminum.
“What you end up with is a semi-product that you can use for whatever you want, and its mechanical properties are improved,” says Wojciech Misiolek, Loewy Professor and Chair of the Department of Materials Science and Engineering and director of the Loewy Institute.
Misiolek, Masashi Watanabe (an associate professor of materials science and engineering), and Natasha Vermaak (an associate professor of mechanical engineering and mechanics) are studying the mechanism behind the breaking and mixing of the oxide into the matrix of aluminum. Doing so would yield benefits beyond reducing the burden on landfills.
“Creating aluminum from recycled materials requires only 5 percent of the energy required to make aluminum out of the ore,” says Watanabe. “We want to reduce that to 2 or even 1 percent. And if we have better properties in these alloys, we’ll need less material for a given use. So we’re talking about a cheaper way to make stronger material. That’s the target.”
Neutralizing NOx
When power plants burn fossil fuels like coal and natural gas, they produce dangerous contaminants like nitrogen oxides (or NOx) that contribute to acid rain, ground-level ozone formation, and greenhouse gases. A common abatement strategy is the selective catalytic reduction (SCR) of nitrogen oxides by ammonia. One such catalyst is titania-supported vanadium oxide.
“The catalyst consists of vanadium oxide and tungsten oxide dispersed on the surface of a titania (TiO₂) support,” says Israel Wachs, the G. Whitney Snyder Professor of Chemical and Biomolecular Engineering. “The vanadium oxide is the active component performing the selective catalytic reduction towards N₂ formation. There’s been a debate in the literature for 40 years over what exactly does the tungsten oxide component do?”
In a paper published in Angewandte Chemie, Wachs describes how he and his team used a High Field (HF) Nuclear Magnetic Resonance (NMR) spectrometer in conjunction with reaction studies to determine the mechanism. “It turns out that the amount of vanadium oxide is very low in the catalyst making the vanadium oxide present as isolated species, or monomers,” says Wachs. “When you add the tungsten oxide, vanadium oxide changes from monomers to oligomers or polymers. We found that these oligomers of vanadium oxide are 10 times more active than in the isolated vanadium oxide sites. So the tungsten oxide changes the structure of vanadium oxide from a less active form to a highly active form.”
This understanding will help guide future designs of SCR catalysts, and will have huge ramifications for industry and air pollution control, he says. “Easily, 40,000 to 50,000 people in the United States die annually due to complications from poor air quality.”
Improving energy storage
The addition of renewable energy into the electrical grid is now forcing conventional power plants to adapt to new power generation realities.
“Fossil power plants once generated power at maximum capacity all day long,” says Carlos Romero, director of Lehigh’s Energy Research Center (ERC). “They were base-loaded, and that’s the best operating point for the plant in terms of thermal efficiency. But with renewables like solar and wind on the grid, these conventional plants now operate on load-following mode and at extremely minimum loads. Because of the deviation, fuel consumption increases, and there is a corresponding monetary loss during the operation of the power plant.”
An interdisciplinary team, promoted by the Institute for Cyber Physical Infrastructure and Energy (I-CPIE), is starting a project on thermal energy storage (TES) for applications in fossil-fired power plants. The group received a three-year, $2 million grant from the Department of Energy to develop an optimized prototype of a “thermal battery.” Their novel concept, called TCM-TES, utilizes engineered cementitious materials enhanced with devices called thermosiphons, which would allow for combined sensible and latent heat storage.
“The fluid inside these devices very quickly changes phases,” says the ERC’s Sudhakar Neti, the project’s principal investigator, “and it is that ability to change phase that enables the heat to move so quickly into and out of the concrete block.”
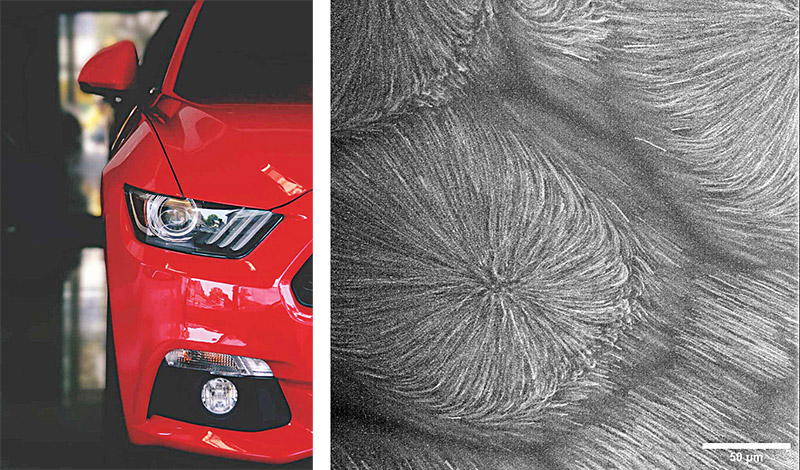 Predicting the drying process
Predicting the drying process
Paint is highly energy intensive to apply, accounting for 60 percent of the energy consumed by a single automotive plant, says James Gilchrist, a professor of chemical and biomolecular engineering.
When an application isn’t done correctly, the vehicle must be recoated and touched up—a process that can cost a single plant more than $10 million per year. Recently, Gilchrist and his collaborators received a grant from the National Science Foundation’s Fluid Dynamics program to better understand paint through kinematics and rheology (the study of how substances flow). “We’re trying to understand the fundamental drying process, and how these paints chemically and physically evolve while they’re drying,” Gilchrist says.
He and his team are using microrheology to study the effect of adding different rheological modifiers—particles with a range of geometric and surface properties—to automotive paint. By putting fluorescent probes in the paint and tracking with a microscope how they move, the researchers can determine if a particular formulation is causing the paint to act like a solid or a liquid as it’s drying.
The goal, says Gilchrist, is to develop a testing method that can be used to both predict how a given formulation will behave, and quickly identify the problem if something goes wrong in the plant.




