Have you seen the cartoon that shows a man standing next to his car at a gas station and aiming the gas nozzle at his head? The sentiment may be popular, but spiraling gas prices are just the tip of the energy crisis. coal and natural gas prices are also up. home heating costs are soaring. deregulation is sending electric bills higher. Meanwhile, concerns grow over pollution and global climate change.
Engineers in the energy field have no shortage of work to do. They are called to develop better ways of generating and distributing energy, to improve the efficiency of systems that consume energy, and to mitigate the environmental impact of energy generation and energy use.
Only by advancing on all three fronts, experts agreed recently at lehigh, can society fi nd solutions to the world’s growing energy problems. Meeting at a lehigh workshop titled “Balancing energy and the environment: an exploration of future Research needs,” the experts forged consensus on a hard truth: There will be no single fix for the world’s energy dilemma. for at least the next two decades, rising global demand will obligate humans to burn coal, oil and natural gas and to build more nuclear power plants, while developing solar, wind, biomass, hydrogen, fusion and other renewable energy sources.
In the area of energy generation and distribution, lehigh engineers focus their energy research in critical areas such as catalysis for efficient hydrogen production, fuel cells, biomass, clean coal technologies, carbon capture and sequestration, solar energy and photovoltaics, and nuclear fusion. in the area of energy consumption and conservation, researchers are working on led lighting, power electronics, energy efficient glass, high-efficiency motors, energy usage auditing and energy-efficient manufacturing.
Meanwhile, in response to the nation’s need for innovation in energy systems engineering, lehigh is partnering with the electric power Research institute (epRi) to launch an integrated research and educational program (p. 16) that will produce the next generation of leaders in energy systems engineering.
The following pages sample a few energy research projects at Lehigh.
Let there be light-energy H2 production
As a fuel, hydrogen (h2) enjoys several key advantages: it emits no greenhouse gases or pollutants, and it can be burned in an engine or used to produce electricity in room-temperature fuel cells.
One barrier to the widespread use of h2 is the lack of a clean, low-energy means of producing it. Much attention, therefore, has been focused on photocatalytic water splitting (pWs) to separate water into oxygen and hydrogen. The field of photocatalysis began in Japan three decades ago.
Ultraviolet (uV) light-active photocatalysts can split water, but scientists are seeking catalysts that react to visible light excitation and utilize the sun’s broad light spectrum more efficiently.
Israel e. Wachs, professor of chemical engineering, is creating and testing structures of titania nanoparticles (nps) to see which can best perform pWs at an industrial scale. Titania (Tio2), a common photocatalyst, is also used in sunscreens because it absorbs uV light.
Wachs is seeking to expand titania’s reactivity from the uV to the visible light range. he and his students conduct tests at oak Ridge national laboratory in Tennessee to measure the lifetime of photo-excited electrons in titania. Their work is funded by the u.s. department of energy (doe).
“Oak Ridge has state-of-the-art spectroscopy equipment,” says Charles Roberts, a ph.d. candidate. “We do fluorescent spectroscopy. When the electron is excited, it emits light energy that can be detected as fluorescence.
“The electron’s lifetime – from ground state to peak excitation back to ground state – lasts a few nanoseconds. it’s important to quantify this. The longer the electron stays excited, the greater the opportunity for titania to perform photocatalysis.
“We measure this phenomenon with spectroscopy with time resolutions in pico and even femtoseconds. By using these extremely high speeds, we’re able to observe the electron’s transitions.”
conventional titania photocatalysts consist of a polymeric network of Tio6 units. The novel titania photocatalysts synthesized by Wachs’ group include isolated Tio4 units, polymeric chains with Tio5 structures, rafts consisting of Tio6 coordination, and Tio6-containing titania nps measuring 1-10 nm. The group is modifying these photocatalytic active sites to optimize the collection of light and the utilization of the formed electrons as well as their corresponding holes, or positive sites. The goal is to establish the fundamental relationships between structure and reactivity for titania-based photocatalysts.
“We want to see if titania’s structure affects the electron’s lifetime,” says Roberts, who has also traveled to France and the Netherlands to work on the project. “We’re trying to determine what’s happening at the molecular level so we can improve titania’s reactivity and efficiency. our ultimate goal is to fi nd the optimal catalyst for splitting clean water into H2 and O2.
“So far, our results show that some isolated Tio4 sites could be superior to the block crystal structure. But although they work well in the lab, we need a lot more catalyst for it to be useful on an industrial scale.”
If successful, Wachs’ group could greatly increase the efficiency of producing H2, says Roberts.
“Photocatalysis runs at very low, even room temperatures. Because light is sufficient to make it go, you are eliminating or greatly reducing your dependence on manmade energy.”
Somphonh Peter Phivilay, another Ph.D. candidate in Wachs’ group, recently spent two months in Japan working with photocatalysis pioneers Kazunari Domen and Masakazu Anpo.
“We tested our catalyst there,” says Phivilay, “and were able to see the relationship between structure and reactivity. We split water and used gas chromatography to measure how many micromoles of H2 we produced. We found isolated surface Tio4 sites to be the most efficient for pWs. The process is not yet cost-effective, but we’re making progress.”
Lowering the heat for H2 production
Steam-methane reforming (SMR), the most common method of producing H2, requires a catalytic reactor heated to almost 900 degrees C, a water-gas shift (WGS) reactor, and a multicolumn, multistep pressure swing adsorption process to purify H2. The process gives off carbon dioxide (CO2) as a by-product.
Shivaji Sircar and Hugo Caram, professors of chemical engineering, have developed and simulated a simpler method of producing fuel cell grade H2 at less than 500 degrees C. Their three-year project is funded by DOE.
The new process, a three-step cyclic thermal swing sorption-enhanced reaction, uses potassium carbonate K2co3-promoted hydrotalcite, a chemisorbent, to selectively remove co2 from the reaction zone of the reformer.
“Our sorbents permit the operation of the SMR reaction at a much lower temperature – approximately 480 degrees C – while still offering more than 90 percent conversion of methane to hydrogen,” says Caram.
“This enables us to directly produce high purity hydrogen that can be used for fuel cells or commercial applications. And we can also separate out pure CO2, which can be sequestered or used for petroleum recovery.”
By utilizing the chemisorbent, says Caram, the researchers are able to integrate the SMR and WGS reactors and the hydrogen-purification unit of the conventional process into a single unit. The chemisorbent is periodically regenerated through pressure or thermal swing adsorption. and removal of CO2 from the reaction zone allows the high conversion of methane to hydrogen.
In another project funded by DOE, Caram and Sircar utilized K2CO3-promoted hydrotalcite and a second sorbent, sodium oxide (NA2O) promoted alumina, to produce fuel cell grade H2 and pure CO2 from synthesis gas. syngas, which contains CO, CO2, H2O and H2, is produced when coal is gasified with steam at high temperatures.
Sircar and Caram reported their results last year in the journal Adsorption and in the International Journal of Hydrogen Energy. They also receive support from the Pennsylvania Infrastructure Technology Alliance and Air products and Chemicals Inc.
Promoting the capture of CO2
Engineers at Lehigh’s Energy Research Center (ERC) develop technologies and systems that increase the efficiency of coal-fired power plants while reducing pollution and facilitating the capture and sequestration of CO2. ErC director Edward K. Levy described some of these efforts in a May 2008 presentation to DOE’s Seventh Annual Conference on Carbon Capture and Sequestration.
One new ERC technology achieves this trio of benefits while also providing a new water source for power plants. The technology, tested successfully at a coal-fired power plant, recovers water vapor from the flue gas with six “staged” heat exchangers whose temperatures are varied so that water vapor and vapors from toxic acids, especially sulfuric acid, condense in separate exchangers. (Water in flue gas condenses at 95 to 130 degrees F, and sulfuric acid at 220 to 310 degrees F.) The recovered water can be treated and used to replace water that evaporates from the cooling tower. Funding for the project is provided by DOE.
Researchers say this technology will be especially useful for power plants in arid regions that lack access to cooling water.
“We were able to recover 50 to 80 percent of the water from the flue gas,” said ERC senior research scientist Harun Bilirgen. “We believe this will make it possible to provide as much as 20 percent of the cooling water needed for a 600-MW power plant boiler burning Powder River Basin coal and 30 percent for the same unit if it’s burning lignite.”
The new design will also improve plant efficiency, reduce mercury emissions 60 to 65 percent and capture sulfuric, hydrochloric and other acids, Bilirgen said. And by cooling flue gas and reducing its acid and water vapor content, the technology could cut the costs of capturing CO2 in back-end amine and ammonia CO2 scrubbers.
“The heat exchanger series will be located along the duct that carries flue gas to the stacks,” said Bilirgen. “The existing temperature of 300 degrees F in this duct will drop to less than 110 degrees F after the exchangers.
“This will be very helpful for post-combustion carbon-capture techniques, which require a fl ue gas temperature of 110 degrees F or lower for efficient operation.”
Shining a laser light on slagging
Slagging – the accumulation of coal ash at high temperatures on the tubes carrying steam in a power plant boiler – costs power plants $2.4 billion a year, according to a recent report by the Electric Power Research Institute (EPRI).
ERC researchers have worked with the energy Research co. (ERCo) of Staten Island, N.Y., to develop an optical technology that lets plant operators make real-time adjustments to prevent slagging and fouling problems. Using laser-induced breakdown spectroscopy (LIBS) and artificial intelligence, the system provides instant analysis of the elemental composition of the coal as it is being burned. LIBS also correlates the fusion temperature of the coal ash, which is affected by the ratio of the elemental ingredients.
The new technology was successfully tested at Brayton Point Station, a coal-fired power plant in Massachusetts. The two-year project was funded by DOE through the New York State Energy Research and Development Authority. The researchers have won a second DOE grant to develop a commercial prototype.
LIBS uses a pulsating laser with two frequencies, one infrared and one visible light. The laser vaporizes a sample and gives a distinct elemental signature represented by intensity and wavelength. from these data, a software package containing artificial neural network models estimates ash-fusion temperature and predicts coal slagging potential.
Traditionally, says ERC associate director Carlos Romero, operators measure coal composition and ash-fusion temperature by taking a sample from a boiler and testing it in a lab. This can take days. Operators can also take measurements with a nuclear analyzer using gamma rays. But the analyzer has a large footprint, says Romero, and is potentially hazardous. LIBS is the size of a tabletop, is relatively safe to use and provides instantaneous data without interrupting the process.
“Our results have been very positive,” says Romero. “LIBS analyzes coal composition accurately and with good repeatability. it also predicts ash-fusion temperature with results that compare very favorably with results obtained using standards of ASTM international.”
Cleaner coal-through drying
half the electricity in the U.S. is generated by coal-fired power plants, but many facilities burn low-rank coals. The moisture content in these coals can approach 40 percent, compared to 6 to 8 percent in high-rank coals, and it adversely affects a plant’s performance and emissions.
engineers at Lehigh’s ERC and at Great River Energy (GRE) in North Dakota have developed a low-temperature coal-drying system that removes moisture from coal using heat rejected by the power plant. The 10-year project was funded by DOE and GRE. GRE is installing the new technology at its 1,160-MW Coal Creek Station, making the station the world’s first to operate with 100 percent feed-dried coal.
Levy and ERC associate director Nenad Sarunac say that at Coal Creek the new technology will remove about a quarter of the moisture from low-rank coal, resulting in a 5 percent improvement in efficiency, a 5 percent reduction in CO2 emissions, and larger reductions in emissions of NOx, SOx and mercury.
The cost of retrofitting Coal Creek with the new technology is relatively low compared to the cost of constructing a new plant that would operate as efficiently, researchers say. given that low-rank coals constitute more than half the world’s coal reserves, the patented technology could potentially be used on a global scale.
ERC researchers are working with U.S. and international companies to integrate the new system into an oxy-combustion power cycle and a coal-to-liquid plant.
The fusion vision
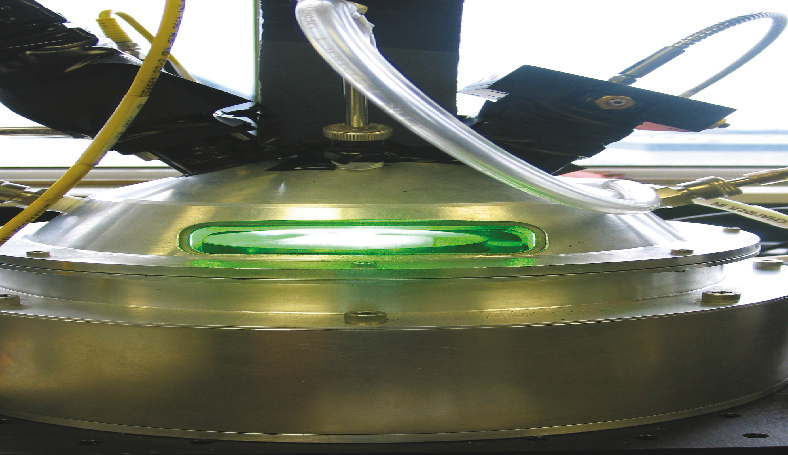
One of the most enticing – and elusive – sources of renewable energy is nuclear fusion, in which isotopes of H2 join under extreme heat (up to 100 million degrees) to create helium atoms. fusion has the potential to provide unlimited supplies of clean, safe energy, says Eugenio Schuster, assistant professor of mechanical engineering and mechanics. It emits no pollutants or greenhouse gases, it produces minimal radioactive waste, and it poses no threat of a large-scale accident.
An international team of engineers and scientists is building ITER, a $10 billion tokamak, or fusion reactor, in France. Their goal is to generate more energy from fusion – five to 10 times more – than is required to heat the hydrogen plasma. Schuster has ties with ITER researchers and with leading U.S. fusion research centers, including general atomics in San Diego and the Princeton Plasma Physics Laboratory. He has organized workshops on fusion for DOE and NSF.
Schuster studies the conditions for a highly confined, stable hydrogen plasma. He seeks to control the shape of the plasma and the radial profiles of plasma variables such as density, temperature and current, while keeping the plasma free of magnetohydrodynamic instabilities.
To understand the evolution of the plasma current profile, schuster models the evolution of the related poloidal magnetic flux profile in normalized cylindrical coordinates. He uses a nonlinear partial differential equation called the magnetic diffusion equation.
By controlling the profile shape of the plasma’s toroidal current, says Schuster, scientists hope to enhance the confinement of the plasma, and the steadiness of the fusion reaction.
DOE regularly funds internships for Schuster’s students at the DIII-D tokamak at general atomics. This year, Yongsheng ou and Chao Xu, two Ph.D. candidates, contributed a paper that was selected as a finalist for the Best Paper Award at the American Control Conference, the largest event of its kind in the U.S.
Experts say it could take 30 years or more before humans enjoy abundant fusion energy, but Schuster is optimistic.
“I’m confident this will be something I can tell my grandchildren about.”