By Christine Fennessy | Photography by Douglas Benedict/Academic Image

// COMPUTER SYSTEMS
Optimizing RDMA for a faster, more robust internet
We are not a species that likes to wait. Especially when it comes to our online demands—we want instant responses to our queries and immediate confirmation of our posts.
Meeting such expectations requires distributed computing systems capable of meeting demand while still preserving the integrity of the data they are providing. Distributed systems enable resource sharing in the form of hardware, software, or data, and comprise multiple machines connected through a network. The internet is the largest, best-known example; others include social networks, online gaming, and e-commerce.
Such systems must perform innumerable complex interactions—fast—for potentially millions of users, without ruining the data.
Improving that speed is at the heart of Roberto Palmieri’s research proposal to optimize the technology known as Remote Direct Memory Access (RDMA) to better serve the massive number of internet-user requests.
RDMA is a fairly recent technology that changed the way computers communicated. At a basic level, that traditional communication involved one machine sending a request to another for a particular service. The second machine had to devote resources to processing and responding to the message, and that all took time. RDMA disrupted that pattern.
“So now, if a machine wants something from another machine, it will not ask for it,” he says. “It will just take it by interacting directly with that machine’s RDMA card. Which means that, instead of spending resources handling the message, the machine can focus on its specific business application. With RDMA, we’re talking about tens of nanoseconds for two machines to interact. Whereas before, we were talking about tens or hundreds of milliseconds. If you’re posting something on social media, and one interaction takes hundreds of milliseconds, and you need 10 interactions, the user is now waiting nearly a second, and starting to think, Why am I waiting so long?”
Palmieri and his team plan to redesign algorithms and protocols to fully exploit the capabilities of RDMA. Everything they produce will eventually become open-source, so others can build on it.
 // CRYSTAL SYNTHESIS
// CRYSTAL SYNTHESIS
A pathway to next-gen semiconductors
Every researcher has their holy grail. For Siddha Pimputkar, it’s cubic boron nitride.
Nitrides are a broad set of chemical compounds in which a nitrogen atom is bonded to another element such as gallium or boron, or most metals.
Some of these nitrides are powerful semiconductors, more efficient than silicon, that ubiquitous presence in just about every device you power on or plug in. Some nitrides rival diamond in their hardness. Some are also capable of working in extreme environments. And some, like cubic boron nitride, can do all those things.
“Compared with silicon, cubic boron nitride has the potential to work under more extreme conditions, including higher voltages and currents,” says Pimputkar. “This allows the elimination or re-envisioning of whole components of circuitry, thereby reducing the size of these power converters, and hence, their cost.”
As a material, nitrides have exciting, far-reaching potential. They’re also really, really hard to make.
But Pimputkar is proposing to do just that. He will attempt to overcome a long-standing problem—the inability to inexpensively grow large, single-crystal nitrides with no defects—and then scale that growth.
His novel solution involves a precursor containing lithium nitride, along with a specialized pressure cooker of sorts capable of containing the highly reactive lithium. Using the same principles you might employ to cook a roast in an Instant Pot, Pimputkar will be able to heat the system to a higher temperature and turn the material into a liquid without the nitride falling apart.
The potential impact, he says, is huge. Pimputkar believes his approach will allow him to make a range of nitride materials, including binary materials like gallium nitride and aluminum nitride (used in visible light LEDs, transistors, and diodes), and ternary materials like aluminum gallium nitride (found in UV LEDs and lasers and UV detectors, among other devices), as well as demonstrate the existence of other novel nitrides. He says it will also allow him to control the activity of each element in the nitride material, giving him control over the composition of the crystal he’s growing. And he’ll be able to grow—for the first time—large-diameter crystals of cubic boron nitride.
“My system is easier to work with, and can be built for larger volumes, so we can now start thinking in terms of industrial scale, say, 6-, 8-, or 10-inch systems. Of course, we will start small, as having even a 1-inch diameter crystal would already prove transformative.”
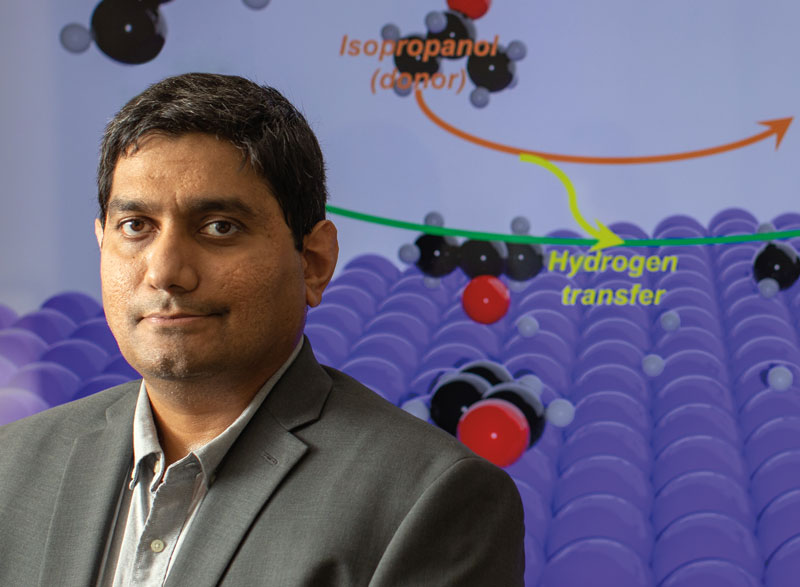 // COMPUTATIONAL PROCESS DESIGN
// COMPUTATIONAL PROCESS DESIGN
Understanding catalytic transfer hydrogenation
Is it possible to build safe, sustainable chemical plants on a small scale? The kind of plants that could—among other things—convert biomass into biofuel, on the very farms producing those crops?
Possibly. But doing so requires figuring out, in part, a more benign process of hydrogenation, the chemical reaction between molecular hydrogen and other compounds and elements that is used to create new molecules.
Srinivas Rangarajan is developing novel tools to better understand a promising chemistry called catalytic transfer hydrogenation (CTH).
“The problem with molecular hydrogen is that it’s very light,” says Rangarajan. “To transport it, you need to compress it using very high pressures. And to use it, you need very high pressures. But if you can use a different molecule that can carry hydrogen chemically, that molecule could be used as a hydrogen donor.
It will decompose or get converted, and in the process, lose hydrogen, which then becomes available for something like biomass conversion.”
The process of transferring hydrogen atoms from a hydrogen donor to a hydrogen acceptor is called catalytic transfer hydrogenation. “So instead of using a molecular hydrogen, you’re using a liquid molecule that can be shipped very easily from where it’s produced to wherever it’s needed,” he explains. “It can then be used at near-ambient temperatures, and at one atmospheric pressure. So in that sense, CTH could lead to a more compact, safe, modular process.”
Rangarajan’s work will attempt to answer two fundamental questions: How does this hydrogen transfer work exactly at the molecular scale, and what’s the right donor and the right catalyst for a given acceptor?
To address possible combinations of donors and catalysts that number in the tens of thousands, Rangarajan will build on his prior research to create a technique called high-throughput kinetic modeling. It incorporates quantum chemistry, optimization, and machine learning to build models that will provide data on how different parameters affect performance.
“This technique allows us to determine how the parameters affect the speed of the reaction and whether the desired product will actually be formed,” he says. “This hasn’t been done before.”
The ultimate goal is to develop processes that are more energy efficient. The potential impact could be huge, he says, given that 45 million tons of hydrogen are used globally every year to make chemicals and fuels.


