It is not often that a large chemical company subjects a commercial catalyst to public scrutiny. Recently, DuPont distributed 3,000 kilograms of a vanadium phosphate-based catalyst (VPO) to more than 20 research groups. In DuPont’s circulating fluidized bed reactor, VPO facilitates the production of maleic anhydride, which is used primarily in the manufacture of polyester resins.
Christopher Kiely, a professor of materials science and engineering, has worked many years with researchers from Cardiff University in the U.K. to characterize and improve the performance of VPO catalysts. The group has found that the most productive form of VPO has a rose-like structure whose active phase is a nanometer-thin disordered layer on the surface of its “petals.” “The ‘rose catalyst’ is intended for a fixed-bed system and would not survive the mechanically abrasive environment of a fluidized bed reactor where the catalyst particles are continuously bounced around,” says Kiely. “When DuPont offered us the chance to examine a commercial VPO catalyst modified to survive those conditions, we jumped at it.”
To improve VPO’s attrition resistance, DuPont scientists spray-dried a slurry of VPO precursor with polysilicic acid. The resulting particles comprise of delicate rosettes of VPO encased in a thin protective spherical shell of porous silica that provides free passage for reactants to enter and products to exit the catalyst.
Using various microscopy techniques, Weihao Weng, a graduate student working with Kiely, discovered that over time, some of the active VPO crystallites were transformed into lessactive phases.
“The more critical observation was that the silica layer became very dense,” says Kiely, “which might inhibit the reactants from reaching the catalyst. This would certainly account for the loss in catalytic activity observed in the used material.”
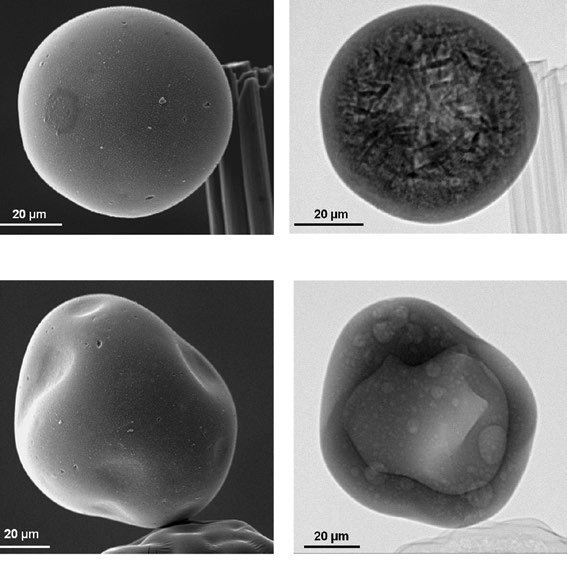
Examination of the particles with Lehigh’s X-ray ultramicroscope (XuM) revealed that not all the particles were actually filled with VPO. Some that appeared to be misshapen in the scanning electron microscope (SEM) images were actually hollow inside.
“This is the first time X-ray ultramicroscopy has been applied to an industrial catalyst,” says Kiely. “The technique allowed us to view the internal structure of individual particles without having to cut them open. This eliminated any concerns about the particles becoming damaged or material falling out of the shell during sample preparation.”