When a blood vessel becomes damaged, platelets come to the rescue. These disc-shaped cells, which are formed in the bone marrow, are the first element in the blood to adhere to damaged endothelial tissue. A subsequent series of biochemical reactions then leads to the production of a fibrin mesh that traps red blood cells and more platelets to form a blood clot.
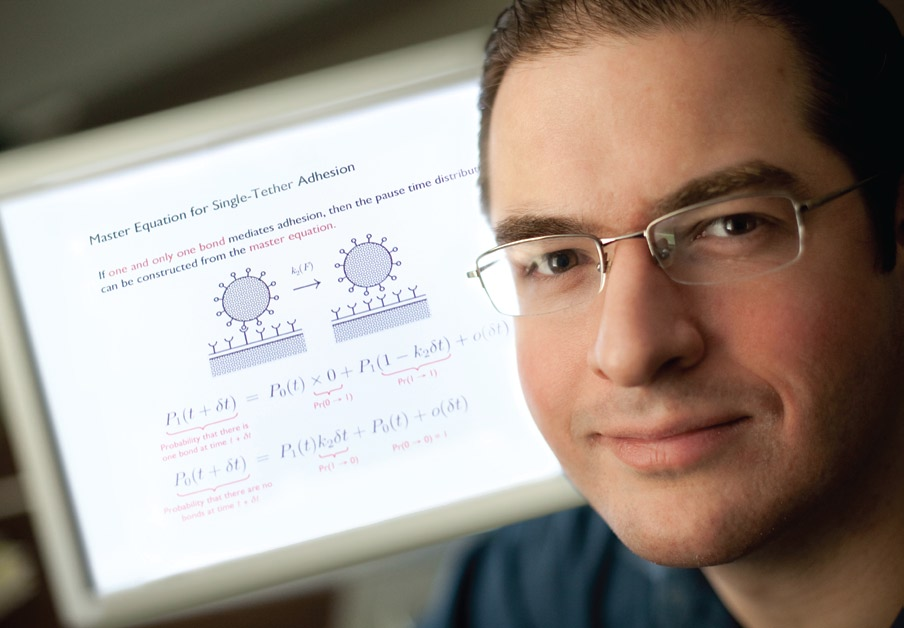
Being able to predict the likelihood of platelet adhesion is the focus of a fundamental computational study conducted by a research group led by Ian Laurenzi, assistant professor of chemical engineering.
A blood platelet measures approximately 2 to 3 microns in diameter. A pint of blood in a healthy adult contains between 70 and 190 billion platelets. The surface of a platelet contains a glycoprotein, which acts as a receptor that can grab onto, react with and form a “tether bond” with a certain type of ligand, or chain of atoms. This is known as the von Willebrand Factor.
As a platelet flows past damaged tissue, the likelihood that it will form a tether bond depends on many factors, including the number of platelets that are passing through the damaged zone, how fast they are traveling (the blood flow rate), and how quickly a bond can form between the platelet receptor and ligand (the chemical reaction rate).
Because most chemical reactions are reversible to some extent, the rate at which the tether bond can be broken must also be considered when determining the likelihood that the bond will form. The number of platelets passing through a damaged area at any one time can also fluctuate significantly; thus, even when a platelet comes into contact with the wound site, formation of a chemical reaction is not guaranteed.
Numerical values for many of these parameters have been gleaned from experiments. For example, the lifetime of tether bond strength under different flow conditions was obtained by using a microscope and a high-speed camera to observe the interaction of platelets with microspheres coated with von Willebrand Factor. This experiment was carried out in a specially designed flow chamber in a collaboration involving Laurenzi and Thomas Diacovo, assistant professor of pediatrics and pathology at Columbia University.
“Most studies so far have looked at only one piece of the puzzle,” says Laurenzi. “Our model takes into account all these factors and incorporates a statistical approach to biochemical kinetics.” The model can account for many variables, such as a change in blood flow rate, alterations to the platelet receptors, and differing platelet concentrations. Its application extends beyond predicting the probability of platelet adhesion and may play a key role in the future development of new clot-controlling drugs.