The catalytic processes used to produce chemicals and fuels could become much more environmentally friendly thanks to a discovery by researchers at Lehigh and Rice Universities.
In an article published Nov. 8 in Nature Chemistry, the researchers reported a novel imaging study of tungstated zirconia that enabled them to design a preparation procedure that increased the activity of the solid acid catalyst by more than 100 times.
Liquid acid catalysts are used to produce chemicals but pose concerns due to evaporation, spilling and corrosion. Solid acid catalysts, a potential replacement, can be more cleanly used and disposed.
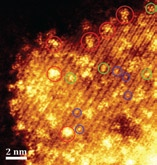
The Lehigh-Rice team used aberrationcorrected scanning transmission electron microscopy and advanced optical microscopy and spectroscopy techniques to illuminate the nanostructure and nanoscale behavior of a tungstated zirconia solid acid catalyst.
The team was able to directly image a variety of tungsten-oxide species that were supported on a nanocrystalline zirconia substrate. Studies revealed that the most active catalytic species were tungsten-oxide clusters that measured 0.8 to 1 nm in diameter and were mixed with a few zirconium atoms emanating from the support.
When the team deposited these clusters onto a tungstated zirconia catalyst with low catalytic activity, the activity of the poor catalyst improved by two orders of magnitude, confirming the team’s hypothesis about the identity and structure of the active species within the tungstated zirconia material.
The Nature Chemistry article's authors include Wu Zhou, a Ph.D. candidate at Lehigh; Israel Wachs, professor of chemical engineering (Lehigh); Christopher Kiely, professor of materials science and engineering (Lehigh); and Michael Wong, associate professor of chemical and biomolecular engineering (Rice).